Search results
Jump to navigation
Jump to search
Page title matches
- ...article could be improved by more references and possibly some words about hydrogen in (micro)biology. And, of course, by facts that I don't know the existence364 bytes (60 words) - 16:54, 3 November 2007
- |elName=Hydrogen '''Hydrogen''' is a [[Chemical elements|chemical element]], typically found as a [[gas]20 KB (3,081 words) - 21:57, 31 March 2022
- |+ Isotopes of Hydrogen and Their Properties441 bytes (54 words) - 10:25, 2 May 2008
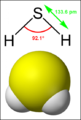
- {{Image|Hydrogen sulfide.png|right|150px|}} ...fide'') is a [[chemical compound]] with the [[chemical formula|formula]] [[Hydrogen|H]]<sub>2</sub>[[Sulphur|S]]. It is a colourless, highly toxic, flammable [6 KB (862 words) - 09:35, 6 March 2024
- 16 bytes (2 words) - 14:07, 9 October 2007
- I know that the foul odor of hydrogen sulfide is commonly referred to as the odor of rotten eggs. I did not inclu Having spent years working in oil refineries, I know what hydrogen sulfide actually smells like ... but I don't know if that is the same odor1 KB (180 words) - 09:27, 6 March 2024
- | pagename = Hydrogen | abc = Hydrogen840 bytes (70 words) - 07:21, 15 March 2024
- Hydrogen's electronegativity is 2.1<ref>http://old.iupac.org/goldbook/E01990.pdf</re193 bytes (22 words) - 16:26, 13 June 2008
- #REDIRECT [[Hydrogen sulphide]]31 bytes (3 words) - 09:27, 6 March 2024
File:Hydrogen sulfide.png (375 × 556 (84 KB)) - 19:53, 11 March 2022- 136 bytes (19 words) - 13:13, 6 July 2008
- 12 bytes (1 word) - 16:54, 3 November 2007
- ...thin green lines from the molecule in the center of the picture represent hydrogen bonds.}} .... Although stronger than most other [[intermolecular force]]s, the typical hydrogen bond is much weaker than both the [[ionic bond]] and the [[covalent bond]].12 KB (1,827 words) - 17:00, 7 March 2024
- 12 bytes (1 word) - 16:11, 27 June 2008
- #REDIRECT [[Hydrogen bond]]27 bytes (3 words) - 11:50, 15 July 2008
- 26 bytes (3 words) - 12:48, 4 June 2011
- #REDIRECT [[Talk:Hydrogen sulphide]]36 bytes (4 words) - 09:27, 6 March 2024
- ...tand how it can explain bonding. (iii) It escapes me why it is stated that hydrogen is a bound state phenomenon. For low enough temperatures all molecular com2 KB (274 words) - 16:57, 3 November 2007
- ...tary charge]]. A better—but never used—name would therefore be hydrogen-like [[cation]]s. ...on orbital#atomic orbital|atomic orbitals]]. The orbitals of the different hydrogen-like atoms differ from one another in one respect only: they depend on the19 KB (2,981 words) - 18:31, 3 November 2021
- 35 bytes (3 words) - 11:25, 23 May 2023
- 454 bytes (73 words) - 17:00, 3 November 2007
- ...nd|covalent]] and non-[[Ionic bond|ionic]] [[chemical bond]] involving a [[hydrogen]] [[atom]] and either [[Fluorine]], [[Nitrogen]], or [[Oxygen]].203 bytes (25 words) - 23:44, 16 July 2008
- ...erful explosive device where the energy is produced through uncontrolled [[Hydrogen fusion]]138 bytes (17 words) - 22:21, 31 March 2022
- #REDIRECT [[Hydrogen/Ground state electron configuration]]58 bytes (6 words) - 13:17, 13 June 2008
- | pagename = Hydrogen sulphide | abc = Hydrogen sulphide2 KB (329 words) - 09:28, 6 March 2024
- 104 bytes (12 words) - 15:23, 17 May 2010
- | pagename = Hydrogen bomb | abc = Hydrogen bomb1,018 bytes (91 words) - 07:28, 25 June 2023
- 84 bytes (10 words) - 22:33, 14 April 2011
- 16 bytes (2 words) - 14:08, 9 October 2007
- 1 bytes (0 words) - 22:41, 9 June 2008
- #REDIRECT [[Talk:Hydrogen/Periodic table of elements]]54 bytes (7 words) - 05:50, 6 March 2024
- A [[chemical compound]] with the [[chemical formula|formula]] [[Hydrogen|H]]<sub>2</sub>[[Sulphur|S]], which is a colorless, highly toxic, flammabl226 bytes (30 words) - 09:47, 6 March 2024
- 244 bytes (33 words) - 05:49, 6 March 2024
- #REDIRECT [[Hydrogen sulphide/Definition]]42 bytes (4 words) - 09:27, 6 March 2024
- An [[atom]], excluding [[hydrogen]] itself, with only one electron, having charge +(Z-1), where Z = atomic nu150 bytes (19 words) - 11:34, 13 July 2008
- | pagename = Hydrogen bond | abc = Hydrogen bond782 bytes (77 words) - 08:39, 15 March 2024
- 35 bytes (5 words) - 03:11, 25 March 2011
File:NASA hydrogen atom.JPG (219 × 144 (11 KB)) - 19:57, 11 March 2022- 12 bytes (1 word) - 16:57, 3 November 2007
- 3 bytes (1 word) - 12:59, 10 June 2008
- * George A. Jeffrey. ''An Introduction to Hydrogen Bonding (Topics in Physical Chemistry)''. Oxford University Press, USA (Mar * A New Intermolecular Interaction: Unconventional Hydrogen Bonds with Element-Hydride Bonds as Proton Acceptor Robert H. Crabtree, Per1 KB (144 words) - 06:45, 13 October 2008
- | pagename = Hydrogen-like atom | abc = Hydrogen-like atom791 bytes (75 words) - 08:38, 15 March 2024
- 1 bytes (1 word) - 17:32, 10 June 2008
- 12 bytes (1 word) - 17:00, 3 November 2007
- <includeonly> </includeonly><noinclude>Hydrogen is in a class of its own, as in some instances it can behave like a metal a165 bytes (28 words) - 05:49, 6 March 2024
- #REDIRECT [[Hydrogen/Periodic table of elements]]49 bytes (6 words) - 08:17, 6 March 2024
- 159 bytes (19 words) - 15:01, 7 December 2008
- ...ergy. In a hydrogen bomb, fusion of deuterium and tritium (two isotopes of hydrogen) releases four times as much energy as the same mass of uranium in a fissio224 bytes (40 words) - 11:25, 23 May 2023
- Hydrogen is sometimes placed at the top of the Halogen group on periodic tables in a295 bytes (51 words) - 05:50, 6 March 2024
- 84 bytes (10 words) - 22:33, 14 April 2011
- {{r|Hydrogen}} {{r|Hydrogen bomb}}173 bytes (20 words) - 11:25, 23 May 2023
- 10 bytes (0 words) - 15:58, 15 June 2008
- 379 bytes (49 words) - 09:27, 6 March 2024
- #REDIRECT [[Hydrogen sulphide/Related Articles]]48 bytes (5 words) - 09:27, 6 March 2024
- 51 bytes (6 words) - 20:13, 18 October 2011
- Auto-populated based on [[Special:WhatLinksHere/Hydrogen bond]]. Needs checking by a human.869 bytes (117 words) - 17:20, 11 January 2010
- 2 bytes (1 word) - 13:17, 13 June 2008
Page text matches
- Hydrogen's electronegativity is 2.1<ref>http://old.iupac.org/goldbook/E01990.pdf</re193 bytes (22 words) - 16:26, 13 June 2008
- ...cause its [[hydrogen]] nuclei are H-1, as opposed to [[heavy water]] whose hydrogen nuclei are H-2.203 bytes (29 words) - 10:21, 12 November 2012
- ...that are H-2, as opposed to regular water, called light water because its hydrogen nuclei are H-1.212 bytes (31 words) - 10:26, 12 November 2012
- ...w.fossil.energy.gov/programs/fuels/hydrogen/currenttechnology.html Today's Hydrogen Production Industry] ...t.org/Files/HydrogenEducation/6HydrogenProductionSteamMethaneReforming.pdf Hydrogen Production – Steam Methane Reforming (SMR)]806 bytes (106 words) - 08:31, 11 September 2023
- [[Hydrogen |<div style="filter:alpha(opacity=99); [[Hydrogen |<div style="-moz-opacity:.99; opacity:.99;width:6px;1 KB (131 words) - 03:39, 22 November 2023
- ...ergy. In a hydrogen bomb, fusion of deuterium and tritium (two isotopes of hydrogen) releases four times as much energy as the same mass of uranium in a fissio224 bytes (40 words) - 11:25, 23 May 2023
- ...article could be improved by more references and possibly some words about hydrogen in (micro)biology. And, of course, by facts that I don't know the existence364 bytes (60 words) - 16:54, 3 November 2007
- #REDIRECT [[Hydrogen bond]]27 bytes (3 words) - 11:50, 15 July 2008
- #REDIRECT [[Hydrogen sulphide]]31 bytes (3 words) - 09:27, 6 March 2024
- #REDIRECT [[hydrogen-like atom]]32 bytes (3 words) - 09:31, 17 September 2007
- #REDIRECT [[hydrogen-like atom]]32 bytes (3 words) - 09:32, 17 September 2007
- #REDIRECT [[Hydrogen sulphide/Definition]]42 bytes (4 words) - 09:27, 6 March 2024
- #REDIRECT [[Talk:Hydrogen sulphide]]36 bytes (4 words) - 09:27, 6 March 2024
- #REDIRECT [[Hydrogen sulphide/Related Articles]]48 bytes (5 words) - 09:27, 6 March 2024
- #REDIRECT [[Hydrogen/Ground state electron configuration]]58 bytes (6 words) - 13:17, 13 June 2008
- #REDIRECT [[Hydrogen/Periodic table of elements]]49 bytes (6 words) - 08:17, 6 March 2024
- #REDIRECT [[Talk:Hydrogen/Periodic table of elements]]54 bytes (7 words) - 05:50, 6 March 2024
- [[Water]] containing the [[isotope]] [[Deuterium]] rather than normal [[hydrogen]]118 bytes (12 words) - 11:07, 6 May 2010
- {{r|Hydrogen}} {{r|Hydrogen bomb}}173 bytes (20 words) - 11:25, 23 May 2023
- Hydrogen isotope information can be found here at http://ie.lbl.gov/education/parent108 bytes (18 words) - 17:10, 13 January 2008
- A class of molecules that contain only [[carbon]] and [[hydrogen]] atoms.110 bytes (14 words) - 19:37, 22 March 2009
- ...cts the existence of a smallest orbit for the [[electron]] circulating the hydrogen [[nucleus]]. Today the radius of this orbit is called the '''Bohr radius' ...constant|Planck's reduced constant]], μ is the [[reduced mass]] of the hydrogen atom (is equal to the [[electron mass]] when the [[proton mass]] may suppos1 KB (231 words) - 08:53, 14 September 2013
- ...cule]] (oxidation); chemical gain of electrons, loss of oxygen, or gain of hydrogen, from and atom, ion, or molecule (reduction)276 bytes (40 words) - 15:52, 1 April 2012
- | pagename = Hydrogen | abc = Hydrogen840 bytes (70 words) - 07:21, 15 March 2024
- ...erful explosive device where the energy is produced through uncontrolled [[Hydrogen fusion]]138 bytes (17 words) - 22:21, 31 March 2022
- An organic molecule that contains exclusively carbon and hydrogen atoms, with only single bonds between carbons147 bytes (19 words) - 15:11, 5 February 2009
- A [[Catalyst|catalytic]] chemical process for converting gaseous hydrogen sulphide into elemental sulphur.142 bytes (16 words) - 09:23, 6 March 2024
- ...nclude>{{Subpages}}</noinclude>Water worlds, larger than Earth, with thick Hydrogen atmospheres, and oceans capable of supporting life138 bytes (18 words) - 08:53, 28 February 2022
- ...ample of pure water, the mass ratio will always be 88.81% oxygen to 11.20% hydrogen. ...mic weights, the fixed atomic ratio of 2-to-1 means that the mass ratio of hydrogen-to-oxygen in any bulk sample of water will be the same.2 KB (342 words) - 19:45, 17 May 2010
- | pagename = Hydrogen-like atom | abc = Hydrogen-like atom791 bytes (75 words) - 08:38, 15 March 2024
- Radius of the first Bohr orbit in the hydrogen atom.88 bytes (13 words) - 08:45, 29 August 2009
- | pagename = Hydrogen bond | abc = Hydrogen bond782 bytes (77 words) - 08:39, 15 March 2024
- *[[Hydrogen]]215 bytes (17 words) - 09:15, 6 March 2024
- ...oactivity|radioactive]] [[isotope]] of the chemical [[elements|element]] [[hydrogen]] containing one [[proton]] and two [[neutron]]s.177 bytes (20 words) - 15:03, 7 December 2008
- An [[isotope]] of the chemical element [[hydrogen]] containing one [[proton]] and one [[neutron]].134 bytes (16 words) - 13:44, 7 July 2008
- An [[atom]], excluding [[hydrogen]] itself, with only one electron, having charge +(Z-1), where Z = atomic nu150 bytes (19 words) - 11:34, 13 July 2008
- The processes for the manufacture of hydrogen (H<sub>2</sub>) and ammonia (NH<sub>3)</sub>.127 bytes (18 words) - 20:30, 25 September 2008
- ...property of a molecule that can transiently bond with water (H2O) through hydrogen bonding.139 bytes (20 words) - 20:25, 3 September 2009
- ...a coolant, that might provide process heat for production of zero-carbon [[hydrogen]] from [[water]].<ref>https://www.gen-4.org/gif/jcms/c_9362/vhtr</ref>196 bytes (31 words) - 02:53, 7 April 2024
- * George A. Jeffrey. ''An Introduction to Hydrogen Bonding (Topics in Physical Chemistry)''. Oxford University Press, USA (Mar * A New Intermolecular Interaction: Unconventional Hydrogen Bonds with Element-Hydride Bonds as Proton Acceptor Robert H. Crabtree, Per1 KB (144 words) - 06:45, 13 October 2008
- ...[[acidic]] substances such as gaseous [[carbon dioxide]] (CO<sub>2</sub>), hydrogen sulfide or [[mercaptan]]s (RSH). Thus, carbon dioxide by itself is an acid Before a raw natural gas containing hydrogen sulfide or carbon dioxide can be used, the raw gas must be treated to reduc2 KB (360 words) - 08:07, 15 March 2024
- | pagename = Hydrogen bomb | abc = Hydrogen bomb1,018 bytes (91 words) - 07:28, 25 June 2023
- ...]] byproduct [[gas]] or any other gas containing significant amounts of [[hydrogen sulphide]] (H<sub>2</sub>S). Natural gas is usually considered sour if the ...amounts of [[acidic]] gases such as [[carbon dioxide]] (CO<sub>2</sub>) or hydrogen sulfide. Thus, carbon dioxide by itself is an acid gas but it is not a sour3 KB (430 words) - 09:44, 6 March 2024
- Currently he is an M.Sc student researching protonated water and hydrogen-bonds (see “Structure and Energetics of the Hydronium Hydration Shells” [[Category:Hydrogen Bond|Markovitch, Omer]]965 bytes (140 words) - 04:29, 22 November 2023
- ...refinery byproduct gas, or any other gas containing significant amounts of hydrogen sulfide (H<sub>2</sub>S).169 bytes (23 words) - 05:46, 3 March 2011
- ...rgy nuclear reactions that occur in metals saturated with deuterium (heavy hydrogen); widely considered to be [[fringe science|fringe]] or [[pseudoscience]].200 bytes (24 words) - 18:19, 20 September 2008
- A [[chemical compound]] (NH<sub>3</sub>) of [[nitrogen]] and [[hydrogen]], occurring as a [[gas]] with a characteristic [[odour]] under [[standard196 bytes (24 words) - 09:18, 18 March 2010
- <includeonly></includeonly><noinclude>Tritium behaves almost exactly like hydrogen in most respects and is therefore difficult to classify as a metal or a non177 bytes (25 words) - 05:50, 6 March 2024
- <includeonly></includeonly><noinclude>Deuterium behaves almost exactly like hydrogen in most respects and is therefore difficult to classify as a metal or a non179 bytes (25 words) - 06:55, 6 March 2024
- A process using aqueous solutions of [[amine]]s to remove [[hydrogen sulphide]] (H<sub>2</sub>S) and [[carbon dioxide]] (CO<sub>2</sub>) from [[187 bytes (28 words) - 09:37, 6 March 2024
- ...monia''' is a [[chemical compound]] (NH<sub>3</sub>) of [[nitrogen]] and [[hydrogen]], occurring as a [[gas]] with a characteristic [[odour]] under [[standard190 bytes (24 words) - 02:00, 13 January 2024
- ...]], that appears to be surrounded by a much larger region of cold, neutral hydrogen.202 bytes (27 words) - 09:12, 1 October 2009
- ...lled with a gas less dense than air or lighter than air (such as helium or hydrogen).166 bytes (28 words) - 23:45, 3 September 2009
- ...sting of two or more molecules held together by van der Waals forces or by hydrogen bonds.159 bytes (24 words) - 04:03, 29 April 2009
- A molecule containing only carbon and hydrogen that exhibits unusual stability and reactivity from having a cyclic conjuga202 bytes (25 words) - 17:38, 2 November 2010
- {{r|Hydrogen-like atom}} {{r|Hydrogen}}777 bytes (99 words) - 16:15, 11 January 2010
- ...ompound that contains the functional group composed of a sulfur atom and a hydrogen atom (-SH).141 bytes (21 words) - 21:06, 3 September 2009
- ...usually slightly cooler than our Sun and often orange in colour; includes hydrogen-burning 'main sequence' stars and older, giant stars such as Arcturus.203 bytes (29 words) - 10:53, 28 October 2011
- Biochemical with an amino group, a carboxyl group, a hydrogen atom, and a side chain bonded to a central carbon.148 bytes (23 words) - 20:58, 5 October 2009
- ..._heating_values.xls Lower and Higher Heating Values of Hydrogen and Fuels] Hydrogen Analysis Resource Center, [[U.S. Department of Energy]]971 bytes (148 words) - 23:47, 23 September 2008
- {{r|Hydrogen bond}} {{r|Hydrogen-like atom}}2 KB (218 words) - 12:57, 15 March 2024
- ...iginally developed for killing insects and rats, a stable preparation of [[hydrogen cyanide]] that, with modifications, was the chemical used in the [[Auschwit241 bytes (31 words) - 21:26, 19 January 2011
- A molecule consisting of an oxygen atom and a hydrogen atom connected by a covalent bond (single bond).140 bytes (21 words) - 20:26, 3 September 2009
- ...a [[rocket engine]] but sometimes in a [[turbine]], such as concentrated [[hydrogen peroxide]] passed over a catalyst that breaks it into steam249 bytes (36 words) - 20:24, 25 March 2010
- A [[chemical compound]] with the [[chemical formula|formula]] [[Hydrogen|H]]<sub>2</sub>[[Sulphur|S]], which is a colorless, highly toxic, flammabl226 bytes (30 words) - 09:47, 6 March 2024
- <includeonly> </includeonly><noinclude>Hydrogen is in a class of its own, as in some instances it can behave like a metal a165 bytes (28 words) - 05:49, 6 March 2024
- ...ace slowly increases, as it mainly takes place at the boundary between the Hydrogen layer and the Helium core. ...nding that a star twice as massive as Sol, our sun, like Sirius, fuses its Hydrogen roughly eight times as fast.1 KB (219 words) - 21:34, 13 April 2022
- '''Zyklon B''' was a stabilized preparation of [[hydrogen cyanide]], originally developed for killing insects and rats, but, with mo The basic preparation, for commercial use, had the hydrogen cyanide adsorbed onto an inert substrate, along with a warning agent that w920 bytes (141 words) - 21:34, 19 January 2011
- A haematological condition in which the reducing hydrogen ion concentration of arterial blood plasma (alkalemia), results in the pH o197 bytes (26 words) - 04:26, 30 September 2009
- ...here most stars - all those in the phase where they shine from energy from Hydrogen fusion in there core, is called the "[[main sequence]]".]] ...gh [[Hydrogen fusion]] - the nuclear process where the nuclei of several [[Hydrogen]] atoms fuse to form an atom of [[Helium]]. Every star above the main sequ3 KB (519 words) - 12:22, 12 April 2022
- ...mistry]] — from the [[chemical element|elements]] [[nitrogen]] and [[hydrogen]].258 bytes (31 words) - 08:37, 4 March 2010
- A chemical compound with one oxygen and two hydrogen atoms (H<sub>2</sub>0). It is often in a liquid form and makes up the bulk214 bytes (36 words) - 03:50, 18 August 2009
- [[Organic compound]]s containing [[carbon]], [[hydrogen]], and [[oxygen]]; includes [[sugar]]s and [[starch]]es that provide [[ener280 bytes (38 words) - 07:50, 7 April 2010
- {{r|Hydrogen-like atom}} {{r|Hydrogen}}1 KB (169 words) - 09:18, 6 March 2024
- ...ation (by synthesis or by other means) of chemical compounds of carbon and hydrogen, which may contain any number of other elements.253 bytes (35 words) - 17:12, 13 May 2008
- ...[[LiH]], and oxygen has an oxidation state of -1 in [[peroxide]]s, e.g. [[Hydrogen Peroxide|H<sub>2</sub>O<sub>2</sub>]];1 KB (248 words) - 12:24, 3 April 2012
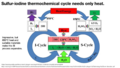
File:Sulfur-Iodine Process.png |description = Sulfur-Iodine Process to make carbon-free hydrogen from water(1,600 × 900 (703 KB)) - 12:56, 19 May 2022- Well-known examples of one-letter symbols are H for [[hydrogen]] and O for [[oxygen]]; For example, H<sup>+</sup> means a hydrogen ion, and Ca<sup>2+</sup> a calcium ion (with two electrons missing).3 KB (478 words) - 05:12, 23 October 2013
- ...at with high core outlet temperatures which enables applications such as [[hydrogen production]] or process heat for the petrochemical industry or others. <ref ...ndustrial plants. Hydrogen generation is one of the major objectives, with hydrogen being seen as a petroleum alternative. Competitive reactors include the [[u2 KB (319 words) - 16:20, 8 January 2023
- {{r|Hydrogen-like atom}} {{r|Hydrogen}}1 KB (143 words) - 10:13, 9 May 2011
- ...nd|covalent]] and non-[[Ionic bond|ionic]] [[chemical bond]] involving a [[hydrogen]] [[atom]] and either [[Fluorine]], [[Nitrogen]], or [[Oxygen]].203 bytes (25 words) - 23:44, 16 July 2008
- ...pound]] with the [[chemical formula|formula]] [[Silicon|Si]]([[Carbon|C]][[Hydrogen|H]]<sub>3</sub>)<sub>4</sub>, used as a standard in <sup>1</sup>H and <sup308 bytes (45 words) - 06:39, 7 April 2010
- |elClass=Periodic table of elements{{!}}Like Hydrogen, Deuterium can behave as a Metal and a Non-Metal ...occurring isotope of hydrogen and represents 0.015% of naturally occurring hydrogen, with H-1 representing the remaining 99.985%.2 KB (287 words) - 05:42, 6 March 2024

File:SUISO FRONTIER left rear view at Kawasaki Heavy Industries Kobe Shipyard October 18, 2020 03.jpg ...ption = 日本語: 液化水素運搬船「すいそ ふろんてぃあ」 左舷後部。2020年10月18日 川崎重工神戸工場にて。 - Liquefied hydrogen carrier "Suiso Frontia" port rear. October 18, 2020 at Kawasaki Heavy Indus(2,560 × 1,707 (929 KB)) - 19:39, 19 April 2022- ...ages}}</noinclude>A theoretical constant related to the spectrum of atomic hydrogen defined by the Bohr theory of the atom as: ''R<sub>∞</sub> = m<sub>e<311 bytes (56 words) - 11:21, 13 March 2011
- ...r of [[organic compound]]s (compounds containing at least one [[carbon]]-[[hydrogen]] bond).336 bytes (45 words) - 00:27, 5 October 2010
- |+ Isotopes of Hydrogen and Their Properties441 bytes (54 words) - 10:25, 2 May 2008
- ...ry powerful gravity, due to its large mass, and is capable of holding onto hydrogen. ...ydrogen when the sun went nuclear 5 billion years ago and blew much of the hydrogen out of the inner [[solar system]]. Saturn's moon [[Titan]] also has an atmo3 KB (509 words) - 11:48, 2 February 2023
- ...h century because of his role as the main developer of the [[fusion device|hydrogen bomb]], his outspoken defense of an unassailable nuclear arsenal, and suppo372 bytes (49 words) - 18:17, 18 June 2009
- ...demonstrator, to try to show that shipping industrial quantities of liquid hydrogen could play a significant role in the world's energy economy.<ref name=kawas The vessel is capable of carrying 1,250 cubic metres of liquid hydrogen in a single sperical insulated double walled tank.<ref name=offshoreEnergyB9 KB (1,054 words) - 13:51, 27 February 2022
- {{r|Hydrogen bond}}190 bytes (27 words) - 07:12, 7 May 2008
- ...ss''' is a process used to produce the useful substance [[ammonia]] from [[hydrogen]] and [[nitrogen]]. ==Sources of hydrogen and nitrogen==7 KB (1,067 words) - 10:08, 28 February 2024
- ...ergy. In a hydrogen bomb, fusion of deuterium and tritium (two isotopes of hydrogen) releases four times as much energy as the same mass of uranium in a fissio1 KB (233 words) - 14:56, 23 May 2023
- {{r|Hydrogen-like atom}} {{r|Hydrogen}}2 KB (229 words) - 09:18, 6 March 2024
- Hydrogen is sometimes placed at the top of the Halogen group on periodic tables in a295 bytes (51 words) - 05:50, 6 March 2024
- ...infin;</sub>'', originally defined empirically in terms of the spectrum of hydrogen, is given a theoretical value by the Bohr theory of the atom as (in [[SI un ...ar=1988 |publisher=World Scientific |author=GW Series |chapter=Chapter 10: Hydrogen and the fundamental atomic constants}}4 KB (708 words) - 17:44, 8 June 2022
- ...gas phase are oxygen and nitrogen (the main components of air), as well as hydrogen and at least four of the halogens (fluorine, chlorine, bromine, iodine, and332 bytes (53 words) - 21:15, 10 November 2020
- {{Image|Hydrogen sulfide.png|right|150px|}} ...fide'') is a [[chemical compound]] with the [[chemical formula|formula]] [[Hydrogen|H]]<sub>2</sub>[[Sulphur|S]]. It is a colourless, highly toxic, flammable [6 KB (862 words) - 09:35, 6 March 2024
- {{r|Hydrogen bond}}339 bytes (43 words) - 11:14, 22 February 2010
- {{r|Hydrogen sulphide}}247 bytes (29 words) - 09:39, 6 March 2024
- ...oes so less readily than carbonic acid. When the bicarbonate ion loses its hydrogen ion, it forms the double-charged negative carbonate ion (CO<sub>3</sub><sup2 KB (371 words) - 01:49, 9 March 2008
- I know that the foul odor of hydrogen sulfide is commonly referred to as the odor of rotten eggs. I did not inclu Having spent years working in oil refineries, I know what hydrogen sulfide actually smells like ... but I don't know if that is the same odor1 KB (180 words) - 09:27, 6 March 2024
- ...is a [[choking gas]] that reacts with water to produce carbon dioxide and hydrogen chloride gas, which is corrosive. Exposure can lead to [[pulmonary edema]] ...px|Phosgene decomposes in the presence of water to form carbon dioxide and hydrogen chloride gas.}}1 KB (194 words) - 12:46, 11 June 2009
- {{r|Hydrogen bond}}436 bytes (51 words) - 00:01, 15 January 2011
- |elClass=Periodic table of elements{{!}}Like Hydrogen, Tritium can behave as a Metal and a Non-Metal ...symbol '''T''' or '''<sup>3</sup>H''', is an [[isotope]] of the element [[hydrogen]] that has a nucleus containing one [[proton]] and two [[neutron]]s (i.e.,1 KB (194 words) - 05:43, 6 March 2024
- {{r|Hydrogen sulphide}}290 bytes (33 words) - 09:52, 6 March 2024
- {{r|Hydrogen sulphide}}295 bytes (35 words) - 09:39, 6 March 2024
- {{r|Hydrogen sulphide}}295 bytes (35 words) - 09:39, 6 March 2024
- {{r|Hydrogen bond}}441 bytes (57 words) - 11:13, 22 February 2010
- {{r|Hydrogen}}196 bytes (24 words) - 22:21, 6 August 2008
- {{r|Hydrogen}}379 bytes (50 words) - 05:22, 3 September 2009
- ...ein]]s. The ''[[side chain]]'', or ''[[residual group]]'' of glycine is a hydrogen atom. It is one of the non-polar amino acids.372 bytes (59 words) - 08:08, 8 June 2009
- {{r|Hydrogen}}400 bytes (49 words) - 21:35, 11 March 2011
- {{r|Hydrogen sulphide}}354 bytes (43 words) - 09:39, 6 March 2024
- ...terize as abnormally increased acidity — measured as pH reduction or hydrogen ion concentration ([H<sup>+</sup>]) increase — accompanied by abnorma558 bytes (73 words) - 15:04, 7 January 2010
- {{r|Hydrogen sulphide}}456 bytes (53 words) - 09:39, 6 March 2024
- {{r|Hydrogen sulphide}}393 bytes (48 words) - 09:39, 6 March 2024
- {{r|Hydrogen bond}} {{r|Hydrogen}}2 KB (280 words) - 09:18, 6 March 2024
- ...ials with a low-Z (i.e., low [[atomic number]], such as [[beryllium]] or [[hydrogen]]) restrict the flow of particles (e.g., [[neutron]]s) while high-Z materia409 bytes (62 words) - 16:30, 11 May 2010
- ...tric density) for several fuels.<ref>https://www.energy.gov/eere/fuelcells/hydrogen-storage</ref>}} ==Hydrogen==6 KB (946 words) - 11:51, 11 April 2023
- ...tand how it can explain bonding. (iii) It escapes me why it is stated that hydrogen is a bound state phenomenon. For low enough temperatures all molecular com2 KB (274 words) - 16:57, 3 November 2007
- {{r|Hydrogen sulphide}}555 bytes (69 words) - 09:39, 6 March 2024
- ...romatic compound]]s with [[chemical element]]s other than [[carbon]] and [[hydrogen]], so they are aromatic but not hydrocarbons. ...ylene]] to give [[ethylbenzene]], which in turn can give [[styrene]] and [[hydrogen]] ( H<sub>2</sub>). Styrene is commonly used as a monomer for polymer5 KB (750 words) - 22:31, 28 November 2012
- {{r|Hydrogen sulphide}}532 bytes (68 words) - 09:39, 6 March 2024
- {{r|Hydrogen}}306 bytes (37 words) - 19:38, 31 May 2010
- ...very energetically when exposed to heat or flame. Methods for extracting hydrogen include electrolysis, biodegradation, sodium sulfate oxidation, carbon free ...rogen gas is then stored in a large collection tank. From that point, the hydrogen can either pass through a [[fuel cell]] and be converted into electricity o9 KB (1,367 words) - 03:34, 22 November 2023
- ...ial is measured in [[volt]]s (V) and is defined relative to the [[standard hydrogen electrode]] (SHE), which is arbitrarily given a potential of 0.00 volts. '' ...s having a positive redox potential; any system donating electrons to the hydrogen electrode is defined as having a negative redox potential. E<sub>h</sub> i3 KB (523 words) - 08:32, 13 February 2009
- ...<sub>2</sub>O''' [[water]] with the isotope [[deuterium]] replacing normal hydrogen. Heavy water has many uses in [[nuclear engineering]], especially as a [[mo550 bytes (85 words) - 11:05, 6 May 2010
- {{r|Hydrogen bond}}454 bytes (58 words) - 16:01, 11 January 2010
- {{r|Hydrogen-like atom}}486 bytes (62 words) - 11:26, 11 January 2010
- ...er]] ''n''. There are ''n''<sup>2</sup> spatial orbitals in a shell; see [[hydrogen-like atom]]s. For instance, the ''n'' = 3 shell contains nine orbitals: one In the case of [[hydrogen-like atom|hydrogen-like]]—one-electron—atoms all orbitals within one shell are de3 KB (430 words) - 13:54, 3 March 2023
- {{r|Hydrogen-like atom}} {{r|Hydrogen}}2 KB (289 words) - 12:57, 15 March 2024
- ...lectron configuration]] and providing a new approach to the placement of [[hydrogen]] and [[helium]].616 bytes (84 words) - 06:33, 6 March 2024
- {{r|hydrogen bond}}449 bytes (54 words) - 12:48, 19 June 2008
- ...as from the feedstock to an [[ammonia production]] plant by contacting the hydrogen sulfide with a bed of solid [[zinc oxide]] (ZnO) with which it reacts to fo ...illation of the ethanolamine solution. However, the reactive absorption of hydrogen sulfide by zinc oxide cannot be reversed. The reactive absorption of carbon4 KB (618 words) - 01:55, 14 March 2024
- ...thin green lines from the molecule in the center of the picture represent hydrogen bonds.}} .... Although stronger than most other [[intermolecular force]]s, the typical hydrogen bond is much weaker than both the [[ionic bond]] and the [[covalent bond]].12 KB (1,827 words) - 17:00, 7 March 2024
- {{r|Hydrogen}}563 bytes (74 words) - 19:22, 11 January 2010
- * A Hydroxide ion is made of one oxygen ion and one hydrogen ion: its chemical formula is (OH)<sup>-</sup>. It has a negative charge. * An [[Ammonium]] ion is made up of one nitrogen atom and four hydrogen atoms: its Chemical Formula is (NH<sub>4</sub>)<sup>+</sup>. It has a posit9 KB (1,524 words) - 10:20, 13 November 2007
- ===Acetylene and hydrogen produced by cracking methane=== For example, if we want to determine the volume of [[acetylene]] and [[hydrogen]] gases produced by cracking 100 grams of [[methane]] gas as per this chemi8 KB (1,289 words) - 22:35, 20 June 2010
- {{r|Hydrogen-like atom}}595 bytes (75 words) - 19:43, 11 January 2010
- {{r|Hydrogen bond}}545 bytes (73 words) - 16:37, 11 January 2010
- ...le-specific subpage]] currently being tested on [[Oxygen]], [[Iron]] and [[Hydrogen]]. It is designed to be a tab-navigable subpage used on elements that have536 bytes (81 words) - 16:01, 22 April 2008

File:Fuel Energy Density.png |author = Hydrogen and Fuel Cell Technologies Office (HFTO), U.S. Dept of Energy |source = https://www.energy.gov/eere/fuelcells/hydrogen-storage(1,518 × 1,422 (1.11 MB)) - 08:04, 31 July 2023- {{r|Hydrogen bond}}709 bytes (91 words) - 19:46, 11 January 2010
- {{r|Hydrogen peroxide}}497 bytes (59 words) - 04:17, 12 September 2013
- {{r|Hydrogen}}677 bytes (90 words) - 20:04, 11 January 2010
- {{r|Hydrogen-like atom}}697 bytes (89 words) - 05:42, 6 March 2024
- ...cids''' are generally defined as those chemical substances which release [[hydrogen]] [[ions]] on dissolving in water. Although acids are most often thought of ...ractically will not come off as H<sup>+</sup> ions, and therefore only the hydrogen on the -COOH group is considered acidic.<br />There is a drawback in this t4 KB (691 words) - 08:05, 15 March 2024
- {{r|Hydrogen-like atom}}702 bytes (87 words) - 20:28, 11 January 2010
- ...'- positions. In duplex DNA, the [[adenine]] base present in adenosine is hydrogen bonded with, that is, it forms a base pair with, a [[thymidine]] nucleotide ...bose ring (see figure). In DNA the 2'-carbon of adenosine is bound to two hydrogen atoms rather than one proton and one hydroxyl group.2 KB (289 words) - 05:19, 17 March 2024
- {{r|Hydrogen bond}}730 bytes (91 words) - 15:58, 11 January 2010
- {{r|Hydrogen bond}}602 bytes (81 words) - 19:38, 11 January 2010
- {{r|Hydrogen}}748 bytes (97 words) - 20:03, 11 January 2010
- {{r|Hydrogen}}802 bytes (101 words) - 12:57, 15 March 2024
- ...equation R-SO<sub>2</sub>-NH<sub>2</sub>, where either of the nitrogenous hydrogen atoms may be replaced by other chemical groups. A large class of [[antibio721 bytes (105 words) - 03:44, 15 November 2010
- {{r|Hydrogen sulphide}}837 bytes (108 words) - 09:39, 6 March 2024
- ...ction provides a combustion analysis for a few typical fuel cases (carbon, hydrogen, sulfur, coal, oil and gas) when the fuel reacts with air at stoichiometric ...that the enthalpy value for basic combustion elements such as carbon (C), hydrogen (H), sulfur (S), oxygen (O) and nitrogen (N) is equal to zero at the standa6 KB (794 words) - 03:50, 22 November 2023
- | pagename = Hydrogen sulphide | abc = Hydrogen sulphide2 KB (329 words) - 09:28, 6 March 2024
- {{r|Hydrogen bond}}571 bytes (79 words) - 21:27, 11 January 2010
- ...lacement of 36 tons, first used a [[hydrogen peroxide]] propulsion system. Hydrogen peroxide, while dangerously reactive, demonstrated significant promise for2 KB (309 words) - 15:42, 8 April 2024
- {{r|Hydrogen}}859 bytes (116 words) - 12:49, 15 March 2024
- ...H<sub>2</sub>O) as an example. The two naturally occurring isotopes of the hydrogen atom are: <br/> The standard atomic weight (isotopically averaged mass) of hydrogen is 1.00794 u.2 KB (363 words) - 21:15, 2 January 2008
- {{r|Hydrogen}}768 bytes (104 words) - 14:34, 10 September 2011
- ...rbon-containing molecules, red, use of ATP, green, used of reducing power (hydrogen). The position of '''biosynthesis''' as a distinct stage in assembly of cel * Reduction equivalents (in the form of hydrogen carried on the coenzymes [[NADH]], [[NADPH]] and others)3 KB (509 words) - 02:33, 8 June 2009
- |molformula= [[Carbon|C]]<sub>9</sub>[[Hydrogen|H]]<sub>13</sub>[[Nitrogen|N]]<sub>4</sub> |molformula= [[Carbon|C]]<sub>9</sub>[[Hydrogen|H]]<sub>13</sub>[[Nitrogen|N]]<sub>4</sub>3 KB (338 words) - 19:37, 22 July 2009
- {{r|Hydrogen-like atom}}876 bytes (107 words) - 10:56, 11 January 2010
- ...bon-carbon) bonds to saturated (single) C—C bonds by addition of [[hydrogen]]. It is also possible to first hydrogenate shark liver oil and then separa813 bytes (121 words) - 07:57, 13 August 2009
- ...|Leadhillite|Lead carbonate|Lead chalcogenide|Lead dioxide|Lead glass|Lead hydrogen arsenate|Lead scandium tantalate|Lead styphnate|Lead tetroxide|Lead zircona1 KB (138 words) - 21:37, 16 June 2008
- ...<sub>2</sub>-C(O)-NH<sub>2</sub>. This side chain is capable of forming [[hydrogen bond]]s with other chemical entities that are electron donors. It is very725 bytes (122 words) - 06:17, 8 June 2009
- *Nitriles are reduced to [[amine|amines]] by [[hydrogen]] and a [[nickel]] catalyst827 bytes (113 words) - 17:46, 28 October 2010
- Auto-populated based on [[Special:WhatLinksHere/Hydrogen bond]]. Needs checking by a human.869 bytes (117 words) - 17:20, 11 January 2010
- ...C<sub>2</sub>H<sub>5</sub>OH is more common for ethanol, to show that one hydrogen and one oxygen combine in a [[hydroxyl]] group.2 KB (350 words) - 10:54, 11 June 2009
- ...]] and myriad [[asteroids]] revolving around it. It mainly consists of [[hydrogen]], which it converts to [[helium]] through a process of [[nuclear fusion]], Hydrogen, about 75%; helium, about 25%; at least 70 other elements make up the remai3 KB (381 words) - 20:54, 21 July 2020
- ...ytosine]] form a very stable Watson-Crick [[base pair]] containing three [[hydrogen bond]]s.932 bytes (125 words) - 18:26, 8 April 2009
- [[Disodium hydrogen phosphite]]<br />2 KB (195 words) - 21:02, 12 May 2008
- {{r|Hydrogen}}1 KB (164 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}1 KB (165 words) - 09:18, 6 March 2024
- ...ding petrol, are primarily a mixture of hydrocarbons (molecules containing hydrogen and carbon molecules) with small amounts of other substances. Crude oil is Petrol is a mixture of compounds of carbon and hydrogen called hydrocarbons; most of the hydrocarbons in petrol are alkenes. In mod2 KB (369 words) - 04:13, 22 November 2023
- {{r|Hydrogen bond}}976 bytes (130 words) - 18:37, 11 January 2010
- ...y, targeting for stabilization the concentration of the positively charged hydrogen ion, [H<sup>+</sup>], a proton, the concentration often expressed in terms Hydrogen ions, protons, in aqueous solution bind to water molecules, molecules of H<2 KB (346 words) - 12:36, 11 January 2010
- {{r|Hydrogen bond}}1 KB (171 words) - 17:03, 8 February 2010
- ===Hydrogen=== Both as ordinary hydrogen and as [[deuterium]], hydrogen moderates neutrons. Most often, it does so in the form of water or heavy wa4 KB (575 words) - 09:51, 8 December 2022
- {{r|Hydrogen-like atom}}1,006 bytes (129 words) - 20:33, 11 January 2010
- ...is of the glycolytic pathway in humans indicates that there are not enough hydrogen ions present in the glycolytic intermediates to produce lactic or any other ...P]] is [[Hydrolysis|hydrolysed]], a hydrogen ion is released. ATP-derived hydrogen ions are primarily responsible for the decrease in pH. During intense exerc4 KB (581 words) - 14:23, 5 November 2007
- {{r|Hydrogen}}1 KB (175 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}1 KB (185 words) - 12:57, 15 March 2024
- ...hydrogen bonds with biological molecules as water molecules are displaced. Hydrogen bonding in aqueous solutions is important for proper protein and DNA functi3 KB (388 words) - 10:27, 13 April 2008
- {{r|Hydrogen}}1 KB (184 words) - 09:18, 6 March 2024
- ...radical formed in radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems ...action with many [[volatile organic compounds]] (VOCs) is the removal of a hydrogen atom forming water and an [[alkyl]] radical (R·).3 KB (416 words) - 14:07, 5 November 2007
- ...ar weaker and reversible noncovalent interactions, such as [[hydrogen bond|hydrogen bonding]], metal coordination, [[hydrophobic effect|hydrophobic forces]], [ ...ized that there were two separate strands of nucleotides connected through hydrogen bonds. The use of noncovalent bonds is essential to replication because the4 KB (497 words) - 11:26, 20 December 2009
- {{r|Hydrogen}}1 KB (184 words) - 09:18, 6 March 2024
- ...f narrative and jargon. To me, this is like telling the joke about the two hydrogen atoms who crossed the street in an article about the Bohr model of the atom899 bytes (155 words) - 06:29, 29 June 2010
- {{r|Hydrogen}}1 KB (185 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}1 KB (185 words) - 09:18, 6 March 2024
- ...a]] (which had a near monopoly on Helium supplies at the time) flammable [[Hydrogen]] was used instead.1,019 bytes (159 words) - 13:19, 2 February 2023
- {{r|Hydrogen-like atom}}1 KB (142 words) - 17:58, 17 April 2010
- {{r|Hydrogen}}1 KB (188 words) - 09:18, 6 March 2024
- ...but it is also part of the solution of the [[Schrödinger equation]] for [[hydrogen-like atom]]s. It is a positive integer (''n'' = 1, 2, 3, …) that ind In the Bohr-Sommerfeld ("old") quantum theory, the electron in a hydrogen-like (one-electron) atom moves in [[Ellipse|elliptic]] orbits. The principa5 KB (873 words) - 15:11, 15 May 2022
- {{r|Hydrogen}}1 KB (189 words) - 09:18, 6 March 2024
- ...ub>2</sub>[[Oxygen|O]]) is a compound whose [[molecule]]s consist of two [[hydrogen]] (H) atoms bonded to one [[oxygen]] (O) atom. Note a 1 after the O is und ...ermediate classes of compounds exist, which feature polar covalent bonds. Hydrogen fluoride is one example.4 KB (657 words) - 11:36, 22 March 2024
- {{r|Hydrogen-like atom}}1 KB (173 words) - 05:43, 6 March 2024
- ...om their environment by electrostatic interactions such as [[Hydrogen bond|hydrogen-bridges]], and so do not disassociate easily.3 KB (469 words) - 18:44, 7 June 2007
- {{r|Hydrogen}}1 KB (194 words) - 12:49, 15 March 2024
- ...ethane is used in the heating of homes and the industrial preparation of [[hydrogen]]. In [[chemistry]], it is the first member of a series of saturated hydroc1 KB (178 words) - 08:34, 8 June 2009
- {{r|Hydrogen}}1 KB (193 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}1 KB (197 words) - 12:49, 15 March 2024
- {{r|Hydrogen}}1 KB (199 words) - 12:57, 15 March 2024
- {{r|Hydrogen}}1 KB (199 words) - 12:57, 15 March 2024
- {{r|Hydrogen}}1 KB (202 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}1 KB (198 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}1 KB (203 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}1 KB (202 words) - 09:18, 6 March 2024
- {{r|Hydrogen-like atom}}1 KB (157 words) - 19:35, 11 January 2010
- {{r|Hydrogen bond}} {{r|Hydrogen}}3 KB (457 words) - 12:49, 15 March 2024
- {{rpl|Hydrogen economy}}1 KB (183 words) - 10:28, 2 April 2024
- ...om their environment by electrostatic interactions such as [[Hydrogen bond|hydrogen-bridges]], and so do not disassociate easily.3 KB (504 words) - 18:45, 7 June 2007
- {{r|Hydrogen}}1 KB (203 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}1 KB (201 words) - 12:57, 15 March 2024
- == Hydrogen bond == ...bond'' and that you started that. I put some comments on the talk page of hydrogen bond, and I like to direct your attention to it and hear your opinion. Than6 KB (923 words) - 04:42, 30 October 2011
- .../nature03383.html ''Ultrafast memory loss and energy redistribution in the hydrogen bond network of liquid H<sub>2</sub>O'']. <br> Letters to Natur1 KB (218 words) - 16:52, 26 August 2010
- ...cle on [[hydrogen-like atom#Quantum numbers of hydrogen-like wavefunctions|hydrogen-like orbitals]] is explained that the angular parts can be designated by le5 KB (822 words) - 17:36, 14 November 2007
- ...m hydrogen (<sup>1</sup>H), and the addition of one or two neutrons to the hydrogen atom forms the isotopes [[deuterium]] (<sup>2</sup>H) or [[tritium]] (<sup>5 KB (829 words) - 21:52, 21 July 2020
- ...problem is that there are many aromatic compounds with elements other than hydrogen and carbon, so not all aromatic compounds are hydrocarbons. I once wr971 bytes (148 words) - 00:24, 23 February 2011
- B. Fiebig, D. Houy, H. Maheshwari, N. Williams. A Self-Regulating Hydrogen-Fueled Flatstack™ Fuel Cell/Li-ion Battery Hybrid Power Source for the Ob1 KB (213 words) - 04:28, 22 November 2023
- {{r|Hydrogen}}2 KB (210 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}2 KB (208 words) - 12:49, 15 March 2024
- {{r|Hydrogen}}1 KB (209 words) - 12:57, 15 March 2024
- {{r|Hydrogen}}2 KB (215 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}2 KB (213 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}2 KB (216 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}2 KB (215 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}2 KB (218 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}2 KB (225 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}2 KB (222 words) - 09:18, 6 March 2024
- ...s a coolant, that might provide process heat for production of zero-carbon hydrogen from water.<ref>https://www.gen-4.org/gif/jcms/c_9362/vhtr</ref>1 KB (194 words) - 18:15, 4 January 2022
- ...ration]], the [[Calvin cycle]] in plants, and through a [[membrane]]-bound hydrogen pump in the [[mitochondria]] called [[ATP synthase]].1 KB (180 words) - 15:17, 17 February 2009
- {{r|Hydrogen}}2 KB (224 words) - 12:49, 15 March 2024
- ...elow the surface. The energy sources for growth of these microbes includes hydrogen gas generated in the interior of the planet, and it is estimated the biomas ...tes that certain microbes can thrive in the absence of sunlight by using [[hydrogen]] gas..."3 KB (470 words) - 14:09, 26 September 2007
- {{r|Hydrogen}}2 KB (211 words) - 09:35, 29 March 2024
- {{r|Hydrogen}}2 KB (232 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}2 KB (226 words) - 09:18, 6 March 2024
- ...[[Niels Bohr]]'s explanation of stationary states of [[hydrogen-like atom|hydrogen atom]]4 KB (522 words) - 10:02, 11 April 2008
- ...increased [[acidity]] — measured as decreased [[pH]] or increased [[hydrogen ion]] concentration (the concentration symbolized as [H<sup>+</sup>]) &mdas ...issociation of carbonic acid, which occurs rapidly spontaneously, yielding hydrogen ions, the bicarbonate concentration also ''increasing'' to abnormally incre7 KB (1,061 words) - 21:55, 11 December 2011
- ...es referred to as the '''''power of hydrogen''''' or the '''''potential of hydrogen'''''. ...p://science.jrank.org/pages/49372/pH.html pH: Potenz, The Determination of Hydrogen Ions, History of Analytical Chemistry, Electrochemistry, Past and Present]11 KB (1,606 words) - 09:39, 29 June 2023
- ...).png | thumb | a hycean world would be a water planet, covered by a thick Hydrogen atmosphere.]] ...would have a thick ocean, where life might be found, covered by a thich [[Hydrogen]] atmosphere.7 KB (854 words) - 22:17, 1 March 2022
- {{r|Hydrogen}}2 KB (231 words) - 09:18, 6 March 2024
- They have different storage lives, from hours to supercooled liquid hydrogen, to years for some stored chemicals. Experienced diesel fuel engineers do n1 KB (204 words) - 08:30, 19 March 2024
- {{r|Hydrogen}}2 KB (250 words) - 09:18, 6 March 2024
- ==Hydrogen== Is Hydrogen considered an Alkali Metal?--[[User:David Yamakuchi|David Yamakuchi]] 15:144 KB (766 words) - 06:33, 6 March 2024
- ...'s atmosphere|air]]) lie below -180 °C while the [[Freon]] refrigerants, [[hydrogen sulphide]], and other common refrigerants have boiling points above − ...named after their inventor, [[James Dewar]], the man who first liquefied [[hydrogen]]. Museums typically display smaller [[vacuum flask]]s fitted in a protect7 KB (1,043 words) - 09:37, 6 March 2024
- {{r|Hydrogen}}2 KB (244 words) - 09:18, 6 March 2024
- ...(primarily aluminium, calcium magnesium, and iron). The lightest elements, hydrogen, helium and lithium, were formed during the [[Big Bang]]. Heavier elements .../sup> in the densest molecular regions. This is about the same as a single hydrogen atom per cubic centimetre or nearly 20 orders of magnitude below that of th7 KB (987 words) - 10:12, 30 May 2009
- ...animal products; the reduction of [[nitrous acid]] and [[nitrite]]s with [[hydrogen]]; and the decomposition of [[ammonium salt]]s by [[alkaline hydroxides]] o The [[Haber process]], which is the production of ammonia by combining hydrogen and [[nitrogen]], was first patented by [[Fritz Haber]] in 1908. In 1910, [11 KB (1,648 words) - 09:02, 4 May 2024
- {{r|Hydrogen}}2 KB (247 words) - 09:18, 6 March 2024
- {{r|Hydrogen}}2 KB (247 words) - 12:57, 15 March 2024
- ...se oxiranes are less stable than other cyclic ethers due to ring strain, [[hydrogen bromide]] (HBr) can be used, without additional heat, to cleave oxiranes su4 KB (546 words) - 17:14, 31 October 2010
- {{r|Hydrogen}}2 KB (245 words) - 17:08, 22 March 2024
- ...animal products; the reduction of [[nitrous acid]] and [[nitrite]]s with [[hydrogen]]; and the decomposition of [[ammonium salt]]s by [[alkaline hydroxides]] o The [[Haber process]], which is the production of ammonia by combining hydrogen and [[nitrogen]], was first patented by [[Fritz Haber]] in 1908. In 1910, [11 KB (1,686 words) - 09:02, 4 May 2024
- ..., [[isobutane]], normal [[butane]], [[nitrogen]], [[carbon dioxide]] and [[hydrogen sulphide]].2 KB (241 words) - 09:37, 6 March 2024
- ...phide]] gas. In [[Petroleum refining processes|petroleum refineries]], the hydrogen sulfide gas is then subsequently converted into byproduct elemental [[sulph ...he vapor phase could be converted into [[saturated]] hydrocarbons by using hydrogen and a catalytic metal. His work was the foundation of the modern catalytic15 KB (2,156 words) - 09:37, 6 March 2024
- {{r|Hydrogen}}2 KB (257 words) - 12:57, 15 March 2024
- {{r|Hydrogen}}2 KB (256 words) - 09:18, 6 March 2024
- ...ne]] (O<sub>3</sub><sup>2</sup><sup>-</sup>, 2.2) but stronger than both [[hydrogen peroxide]] (H<sub>2</sub>O<sub>2</sub>, 1.8 V) and [[permanganate]] (MnO<su2 KB (234 words) - 21:11, 27 April 2011
- {{r|Hydrogen}}2 KB (263 words) - 09:18, 6 March 2024
- ...volume by water vapor (H<sub>2</sub>0) created by the combustion of the [[hydrogen]] in the fuel with atmospheric oxygen. Much of the 'smoke' seen exiting fr ...ural gas produces more water vapor than does the burning of coal since the hydrogen-to-[[carbon]] ratio of natural gas is higher than that of coal.4 KB (619 words) - 09:16, 6 March 2024
- {{chem|[[Hydrogen|H]]|2|[[Oxygen|O]]}} : {{chem|[[Hydrogen|H]]|2|[[Oxygen|O]]}}4 KB (486 words) - 11:49, 19 January 2023
- ...SO<sub>4</sub><sup>-</sup> is a medium strength acid from which the second hydrogen dissociates to form the ''[[sulfate]]'' anion SO<sub>4</sub><sup><small>-2<6 KB (957 words) - 05:47, 12 September 2013
- ...phide]] gas. In [[Petroleum refining processes|petroleum refineries]], the hydrogen sulfide gas is then subsequently converted into byproduct elemental [[sulph ...he vapor phase could be converted into [[saturated]] hydrocarbons by using hydrogen and a catalytic metal. His work was the foundation of the modern catalytic15 KB (2,197 words) - 09:37, 6 March 2024
- {{r|Hydrogen bond}}2 KB (246 words) - 16:13, 1 April 2011
- ...cale uses the [[Haber process|Haber-Bosch process]], in which nitrogen and hydrogen gasses react, in the presence of iron, heat and pressure, to produce ammoni ...ause the molecule has a pyramidal structure, it is very polar and can form hydrogen bonds with water so that saturated aqueous ammonia is 15 M.3 KB (539 words) - 18:53, 5 January 2021
- {{r|Hydrogen bond}}2 KB (265 words) - 10:53, 11 January 2010
- ...l]]s that exhibit [[alpha-tocopherol]] activity. by virtue of the phenolic hydrogen on the 2h-1-benzopyran-6-ol nucleus, these compounds exhibit varying degree1 KB (193 words) - 03:28, 6 January 2011
- ...for both the actinides and other metals such as [[ruthenium]]. The dibutyl hydrogen phosphate can make the system behave in a more complex manner as it tends t4 KB (649 words) - 13:03, 15 March 2024
- |elName=Hydrogen '''Hydrogen''' is a [[Chemical elements|chemical element]], typically found as a [[gas]20 KB (3,081 words) - 21:57, 31 March 2022
- ...condary structure]] and by the overall tertiary structure of the protein. Hydrogen bonding also plays a significant role in a protein's structure, especially ...kbone atoms form a coil (black bonds) while the carbonyl groups (red) form hydrogen bonds with the amide groups (blue)}}9 KB (1,340 words) - 22:09, 11 February 2010
- ..., [[jet fuel]] and [[diesel oil]]. The process takes place in a [[Hydrogen|hydrogen-rich]] atmosphere at elevated [[temperature]]s (260 – 425 [[Celsius|°C] ...ing feedstock are, to a large extent, also hydrogenated and form gaseous [[hydrogen sulphide]] (H<sub>2</sub>S) and [[ammonia]] (NH<sub>3</sub>) which are subs17 KB (2,523 words) - 09:37, 6 March 2024
- ...s received on Earth. Observations focus on the 1420 MHz frequency at which hydrogen radiates, knowledge of which is believed to be a prerequisite for any civil2 KB (221 words) - 18:30, 25 October 2010
- # [[Hydrochloric acid]] (including anhydrous hydrogen chloride)2 KB (228 words) - 14:47, 5 June 2008
- ...al energy to carry out the process but will yield higher amounts of actual hydrogen and lower amounts of by-products. Gasification involves four main steps, d ...Trifiro, A Vaccari, “The technical feasibility of biomass gasification for hydrogen production” Catalysis Today, Volume 106, Issue 1-4, 297-300, Oct. 15, 2007 KB (1,025 words) - 03:46, 22 November 2023
- ...the mass of our Sun can continue to burn each element synthesised in turn, hydrogen, helium, then [[carbon]], [[oxygen]], [[silicon]] and so forth until they a ...hemselves. This mass deficiency is different from element to element, with hydrogen having a very low deficiency. Iron has the highest deficiency of all elemen4 KB (664 words) - 16:43, 28 November 2010
- Red dwarfs [[Nuclear fusion|fuse]] [[hydrogen]] to [[helium]] via the [[Pp chain|proton-proton (PP) chain]]. Due to the l ...ed dwarfs are fully convective, they can burn a larger proportion of their hydrogen before leaving the [[main sequence]] than larger stars, such as our sun. Re7 KB (1,111 words) - 11:24, 30 July 2022
- ...xis. They are joined together in pairs, a single base from one chain being hydrogen-bonded to a single base from the other chain" ...relative contributions are 33.4% base stacking, 30.3% van der Waals, 18.2% hydrogen bonding, 12.1% hydrophobic, and 6.1% electrostatic"5 KB (816 words) - 01:14, 8 July 2007
- ...r of [[organic compound]]s (compounds containing at least one [[carbon]]-[[hydrogen]] [[covalent bond]]).<ref>[http://www.science.uwaterloo.ca/~cchieh/cact/app ...sidered to be an organic compound even though it does not contain a carbon-hydrogen bond.13 KB (1,921 words) - 09:37, 6 March 2024
- ...inorganic chemistry is that it does not include compounds having a carbon-hydrogen bond ( C-H bond). For example ( as listed in the main article) the carbon e ...vast number of organic compounds (compounds containing at least one carbon-hydrogen chemical bond)."8 KB (1,192 words) - 02:56, 9 October 2010
- ...one-electron Schrödinger equation for an atomic electron. In the case of [[hydrogen-like atom|one-electron atom]]s the Schrödinger equation is exactly solvab ...bitals]] (STOs) or [[Gaussian type orbitals]] (GTOs). [[Hydrogen-like atom|Hydrogen-like orbitals]] are rarely applied in numerical calculations, because they14 KB (2,265 words) - 05:37, 6 March 2024
- ..., superimposed on each other in various proportions. The angular parts of hydrogen-like orbitals are used as a basis for simple schemes of hybridisation, beca ...e carbon should have 4 orbitals with the correct symmetry to bond to the 4 hydrogen atoms. The problem with the existence of methane is now this: Carbon's [[g14 KB (2,154 words) - 09:32, 12 November 2007
- ...equivalents of hydroxide, with the general formula R2N-(where R refers to hydrogen or any alkyl group."2 KB (230 words) - 06:50, 1 November 2010
- ...te, reactive metal. Potassium reacts violently with [[water]], producing [[hydrogen]]. With a density of 0.862 g/cc, potassium is less dense than water.2 KB (246 words) - 22:19, 11 January 2021
- ...Eckener was successful in lobbying the U.S. government for the purchase of Hydrogen but ruled it out on financial grounds. ...aith in the security of dirigibles was shattered, and flying passengers in hydrogen-filled vessels became untenable. LZ 127 ''Graf Zeppelin'' was retired one m6 KB (973 words) - 10:23, 8 April 2023
- {{r|Hydrogen bond}}2 KB (271 words) - 07:01, 9 September 2010
- ...that an electron orbit is not a trajectory, but a stationary state of the hydrogen atom. ...ouds than planetary orbits. As a matter of fact, the angular parts of the hydrogen wave functions are spherical harmonic functions and hence they have the sa10 KB (1,514 words) - 19:38, 20 November 2009
- ...tary charge]]. A better—but never used—name would therefore be hydrogen-like [[cation]]s. ...on orbital#atomic orbital|atomic orbitals]]. The orbitals of the different hydrogen-like atoms differ from one another in one respect only: they depend on the19 KB (2,981 words) - 18:31, 3 November 2021
- ...K (−222 °C), and for [[hydrogen]], about 202 K (-71 °C). Thus, helium and hydrogen will warm during a J-T expansion at typical room temperatures. On the othe ...is reason, a simple Linde cycle cannot normally be used to liquefy helium, hydrogen and [[neon]].7 KB (1,081 words) - 05:42, 4 September 2013
- *[http://www.abc.net.au/science/k2/moments/s1052864.htm Hindenburg & Hydrogen]3 KB (410 words) - 10:01, 28 September 2013
- ...K (−222 °C), and for [[hydrogen]], about 202 K (-71 °C). Thus, helium and hydrogen will warm during a J-T expansion at typical room temperatures. On the othe ...is reason, a simple Linde cycle cannot normally be used to liquefy helium, hydrogen and [[neon]].7 KB (1,084 words) - 05:41, 4 September 2013
- ...a hydrogen bond with one of the amino protons (H61) of adenine. A second hydrogen bond is formed between the uracil H3 proton and the adenine N1 nitrogen ato4 KB (593 words) - 13:27, 19 June 2008
- ...called ''hydrodesulfurization'' which converts the sulfur compounds into [[hydrogen sulphide]] [[gas]] that is removed from the naphtha by [[Continuous distill ...gas processing]] plants (which also use amine gas treating units to remove hydrogen sulfide from the raw [[natural gas]]).<ref>[http://minerals.usgs.gov/minera9 KB (1,344 words) - 09:37, 6 March 2024
- ...ry, it was discovered that atomic masses are not integral multiples of the hydrogen mass; now we understand that this is because isotopically averaged masses w ...tely understood. In 1913 protons (which, after all, are nothing but atomic hydrogen [[cation]]s) were well-known, but the [[neutron]] still had to be discovere7 KB (1,066 words) - 05:40, 6 March 2024
- An [[inductor]] creates a strong magnetic field around a [[hydrogen]]-rich fluid, causing the protons to align themselves with the newly create ...en a special liquid (containing free, unpaired electrons) is combined with hydrogen atoms and then exposed to secondary polarization from a radio frequency (RF9 KB (1,370 words) - 08:18, 12 September 2013
- *[[Acid gas]]es: [[carbon dioxide]] (CO<sub>2</sub>), [[hydrogen sulphide]] (H<sub>2</sub>S) and [[mercaptan]]s such as [[methanethiol]] (CH *Contain no more than trace amounts of components such as hydrogen sulfide, carbon dioxide, mercaptans, nitrogen, and water vapor.11 KB (1,750 words) - 09:37, 6 March 2024
- ...her than capitalized (or should we just rename the article to Potential of hydrogen)? Wikipedia seems to use a {{tl|lowercase}} template that we don't have. [[ ...fective concentration) of hydrogen ions, but not just the concentration of hydrogen ions. However, I do not know how to modify the formula, can anyone correct7 KB (1,174 words) - 04:47, 2 March 2011
- |hazard=Boron hydrogen compounds (boranes) are toxic as well as highly flammable ...s and react readily with water to produce boric acid and the corresponding hydrogen halide.5 KB (804 words) - 19:43, 31 December 2020
- ...rious [[alkanol]]s (commonly referred to as simply [[amine]]s) to remove [[hydrogen sulphide]] (H<sub>2</sub>S) and [[carbon dioxide]] (CO<sub>2</sub>) from [[ ...lt in products which no longer have the sour, foul odors of mercaptans and hydrogen sulfide.9 KB (1,476 words) - 09:37, 6 March 2024
- ...rious [[alkanol]]s (commonly referred to as simply [[amine]]s) to remove [[hydrogen sulphide]] (H<sub>2</sub>S) and [[carbon dioxide]] (CO<sub>2</sub>) from [[ ...lt in products which no longer have the sour, foul odors of mercaptans and hydrogen sulfide.9 KB (1,470 words) - 09:37, 6 March 2024
- ...''' is a [[Catalyst|catalytic]] chemical process for converting gaseous [[hydrogen sulphide]] (H<sub>2</sub>S) into elemental [[sulphur]] (S).<ref name=Gary>{ ...[[natural gas]] and from the by-product [[sour gas|sour gases]] containing hydrogen sulfide derived from refining [[petroleum crude oil]] and other industrial13 KB (1,990 words) - 09:37, 6 March 2024
- ...as both a moderator and a coolant to carry away heat simultaneously. The hydrogen in such light water is practically 100% H-1. A nuclear reactor which uses ...st nuclei are in hydrogen-2 (<sup>2</sup>H, H-2, or D, also called ''heavy hydrogen'' or ''[[deuterium]]'') atoms and moderate neutrons for fission quite well10 KB (1,554 words) - 14:19, 24 January 2023
- == About [[Hydrogen sulphide]] == David, if you have the time, I would appreciate it if you reviewed [[Hydrogen sulphide]]. It could use some more content. [[User:Milton Beychok|Milton Be8 KB (1,293 words) - 09:41, 6 March 2024
- ...called ''hydrodesulfurization'' which converts the sulfur compounds into [[hydrogen sulphide]] [[gas]] that is removed from the naphtha by [[Continuous distill ...gas processing]] plants (which also use amine gas treating units to remove hydrogen sulfide from the raw [[natural gas]]).<ref>[http://minerals.usgs.gov/minera10 KB (1,443 words) - 09:37, 6 March 2024
- See [[hydrogen-like atom]] for a further explanation of this notation. See the article [[e <tr><td>1 <td>H <td>[[Hydrogen]] <td bgcolor="#D0D0D0">1 <td>21 KB (3,868 words) - 09:15, 6 March 2024
- ...hylene and methane, and found that methane contained exactly twice as much hydrogen as ethylene. Why this mathematical simplicity? <ref name=jaffe76>Jaffe B. ( ...tomic weights of different elements — relative to the unit weight of hydrogen.11 KB (1,717 words) - 18:42, 4 January 2012
- ...e hydrocarbon molecules and produces very significant amounts of byproduct hydrogen [[gas]] for use in a number of the other processes involved in a modern pet ...that use [[methanol]] or [[biomass|biomass-derived]] feedstocks to produce hydrogen for [[fuel cells]] or other uses.19 KB (2,771 words) - 09:16, 6 March 2024
- ...e hydrocarbon molecules and produces very significant amounts of byproduct hydrogen [[gas]] for use in a number of the other processes involved in a modern pet ...that use [[methanol]] or [[biomass|biomass-derived]] feedstocks to produce hydrogen for [[fuel cells]] or other uses.19 KB (2,792 words) - 09:16, 6 March 2024
- ...the temperature is raised to above 90° C. Due to the high temperature, the hydrogen bonds between the two strands of DNA break and the strands are separated. N2 KB (331 words) - 07:01, 17 August 2016
- {{r|Hydrogen}}2 KB (323 words) - 12:57, 15 March 2024
- ...urs. Energy for transportation could be electrical for light vehicles, and hydrogen for larger vehicles and rail. Synthetic fuel could be generated for airplan ...ion for storage in a few favorable locations. Batteries are too expensive. Hydrogen may be possible, but the technology is still being developed. See [[Energy9 KB (1,503 words) - 15:56, 17 July 2023
- ...e ions. Keep in mind the charge-distribution within the water molecule. So hydrogen-bonding is the cause of the strange behavior of water as a liquid: having i ...rent time, is not practiced on a commercial large-scale as a source of the hydrogen used to produce ammonia.4 KB (741 words) - 04:10, 2 March 2010
- ...agram of chemical equation for converting methylcyclohexane to toluene and hydrogen''' ...''Diagram of chemical equation for converting <br>n-heptane to toluene and hydrogen'''||[[Image:CatReformerEq3.PNG|300px]]<BR><BR>'''Diagram of chemical equati9 KB (1,226 words) - 04:25, 22 November 2023
- ...diation. An example is the conversion of water into [[hydrogen]] gas and [[hydrogen peroxide]]. ...of organic compounds has been reported. For instance the use of radiogenic hydrogen peroxide (formed by irradation) to remove sulfur from [[coal]] has been rep12 KB (1,939 words) - 12:51, 15 March 2024
- ...carbons''' are a class of [[molecule]]s that contain only [[carbon]] and [[hydrogen]] atoms. Some of them make very good fuels. [[Gasoline]] contains a mixtu ...s as the number of carbon atoms, with each carbon atom being bonded to two hydrogen atoms and to two other carbon atoms. They are referred to as '''cycloalkane8 KB (1,213 words) - 11:30, 2 November 2010
- ...known as [[allotrope]]s of carbon. Most organic carbon compounds contain [[hydrogen]]; those that contain [[oxygen]] as well include the extensive class of [[c5 KB (806 words) - 17:16, 1 January 2021
- ...e, which I will have to get to later. First, almost all compounds (except hydrogen!) What do you mean by "First, almost all compounds (except hydrogen!)14 KB (2,299 words) - 08:35, 6 March 2024
- ...s using quantitative numbers relating to the potential explosive energy of hydrogen that could be rapidly generated from contact of 5000 tons of reactive, neut ...causing the rapid generation of hydrogen and a very powerful and energetic hydrogen detonation.<br>7 KB (1,065 words) - 11:05, 7 July 2023
- ...ailable), and ammonia fertilizer production (from nitrogen in the air, and hydrogen from water electrolysis)2 KB (331 words) - 04:50, 22 November 2023
- ...of two or more molecules held together by [[van der Waals forces]] or by [[hydrogen bonds]]. The name originated in the beginning of the 1970s when stable mole2 KB (310 words) - 17:14, 15 November 2007
- ...[[Star|stars]], with each star containing about 10<sup>57</sup> atoms of [[hydrogen]]. ...]] and 4% [[atoms]]. Thus the density of atoms is on the order of a single hydrogen [[nucleus]] (or atom) for every four cubic meters of volume. The exact natu8 KB (1,199 words) - 20:34, 8 June 2010
- *[[Electrolysis]] of water to form [[hydrogen]] (H<sub>2</sub>) and [[oxygen]] (O<sub>2</sub>) [[gas]]es (absorbs electri :[[Nitrogen|N]]<sub>2</sub> + 3 [[Hydrogen|H]]<sub>2</sub> → 2 [[Ammonia|NH<sub>3]]</sub>11 KB (1,592 words) - 09:15, 28 September 2013
- ...ation step, as illustated in the figure. In the first oxidation step, the hydrogen on the sulfur atom is replaced with an [[hydroxyl]] group (OH), creating a3 KB (353 words) - 11:31, 11 December 2010
- ...he hydrogen line, and the second being about 0.0498 MHz more than the hydrogen line. The [[Bandwidth (signal processing)|bandwidth]] of the signal is less12 KB (1,817 words) - 22:07, 30 April 2013
- In the laboratory, it can be made by reacting [[sodium hydrogen sulfide]] with a strong acid, such as sulfuric acid.2 KB (354 words) - 09:16, 6 March 2024
- ..., general route to <math>\alpha</math>-lactam (aziridinone) products. The hydrogen gas and sodium halide by-products are readily removed.<ref>{{cite journal|j2 KB (362 words) - 17:14, 21 March 2024
- ...specific, hence the ''selective'' description. The most common type is a hydrogen-sensing electrode used in [[pH]] measurements. They are increasingly common2 KB (343 words) - 09:43, 3 March 2011
- | align="center"|[[Hydrogen]] <ref name=Perry>{{cite book|author=Perry, R.H. and Green, D.W. (Editors)| .../hydrogen/datasheets/lower_and_higher_heating_values.xls Heating Values of Hydrogen and Fuels] [[U.S. Department of Energy]]</ref>13 KB (1,833 words) - 05:42, 19 October 2013
- ...l reactivity is quite low, but can tarnish in the presence of [[ozone]], [[hydrogen sulphide]], or air mixed with [[sulphur]].<ref name=LANL/>2 KB (332 words) - 09:37, 6 March 2024
- ...ode]] where instead of a metal [[anode]] reacting and dissolving it is the hydrogen gas which is consumed.8 KB (1,357 words) - 12:52, 15 March 2024
- ...euss. Akad. d. Wiss.</ref> undertook measurements on excited states of the hydrogen atom and succeeded in observing splittings. ...ass Wasserstoffspektrum vom Standpunkt der neuen Quantenmechanik'' (On the hydrogen spectrum from the point of view of the new quantum mechanics). Zeitschrift13 KB (2,036 words) - 18:38, 10 February 2010
- {{tl|Selected Electronegativities|Hydrogen|Beryllium|Iron|Helium|Uranium|Neptunium|Lead}} {{tl|Selected melting points|Hydrogen|Beryllium|Iron|Helium|Uranium|Neptunium|Lead|Cobalt(II) oxide}}5 KB (819 words) - 05:42, 6 March 2024
- ...he evening of March 28. By March 30, there were new concerns about a large hydrogen bubble which had formed inside the reactor and seemed to be in danger of br2 KB (371 words) - 10:43, 8 April 2024
- ...the cost of [[Energy storage|storage]]. The most promising possibility is hydrogen at $0.12 per kWh (see below). Assuming this cost is distributed evenly over ===Hydrogen:===18 KB (2,685 words) - 10:33, 15 May 2024
- 1 H [[Hydrogen]] · 1 H [[Hydrogen (element)|Hydrogen]] ·14 KB (1,456 words) - 09:15, 6 March 2024
- ...ful. For instance, the normalizable eigenstates of a [[hydrogen-like atom|hydrogen-like]] Hamilton operator do not span the whole vector space that they belon8 KB (1,273 words) - 11:29, 9 July 2009
- The unit ''u'' is convenient because one [[hydrogen]] atom has a mass of approximately 1 ''u'', and more generally an [[atom]] ...in the early nineteenth century, who introduced the mass of one atom of [[hydrogen]] as the atomic mass unit. Later [[Francis Aston]], inventor of the [[mass7 KB (1,035 words) - 13:02, 11 September 2011
- ...onsider the best approximation. A famous example is the description of the hydrogen atom with a single Gaussian variation function with one non-linear variatio2 KB (377 words) - 10:50, 12 May 2009
- ...explain certain regularities that were observed in the spectra of atomic [[hydrogen]] and [[helium]]. Initially they thought that electrons rotate physically a2 KB (357 words) - 10:17, 8 April 2023
- ...d by using fuel with a high initial density (highly compressed gas, liquid hydrogen, or lithium hydride), by efficient compression during implosion, or most li ...the form of a metal hydride powder, uranium hydride (UH3) for example. The hydrogen can be rapidly and efficiently released by heating the hydride to a high te16 KB (2,501 words) - 03:57, 22 November 2023
- ...e]], with the general formula R<sub>2</sub>N<sup>-</sup>(where R refers to hydrogen or any alkyl group). These are the conjugate bases of amines, and have pK<s3 KB (410 words) - 02:51, 17 October 2013
- :[[hydrogen]] (H<sub>2</sub>) : 1282.1 L·atm/mol ...with the example numerical values presented for oxygen, carbon dioxide and hydrogen and with their corresponding dimensional units.11 KB (1,729 words) - 05:20, 3 September 2013
- ...menolytic agents. These may be water-based (such as saline, acetic acid or hydrogen peroxide), oil-based (not true cerumenolytics), or other. No type is clearl2 KB (377 words) - 11:46, 2 February 2023
- *[[Hydrogen sulphide]] (H<sub>2</sub>S} ...h and low pressure separators will probably need to be processed to remove hydrogen sulfide before the water can be disposed of or reused in some fashion.7 KB (1,134 words) - 09:41, 6 March 2024
- ...ce shell is in a so-called ''ns''-[[Hydrogen-like atom#Quantum numbers of hydrogen-like wave functions|orbital]]. In all these metals there is only one free e ...ents in that same group (column) will react. For instance [[carbon]] and [[hydrogen]] (H<sub>2</sub>) react to form [[methane]]. The bonds of the [[Carbon|carb13 KB (2,075 words) - 09:16, 6 March 2024
- (3) the transfer of an electron covalently bonded to a proton, namely, a hydrogen atom, from one chemical species to another. Here the hydrocarbon, [[ethane]] (H<sub>3</sub>C-CH<sub>3</sub>) loses two hydrogen atoms, hence two electrons, to form [[ethene]], an [[alkene]], the ethane u16 KB (2,492 words) - 16:30, 7 August 2012
- [[Hydrogen]]/[[air]] 2000-2100<br/> [[Hydrogen]]/[[oxygen]] 2550-27006 KB (888 words) - 13:04, 12 April 2011
- :<font color=green>Futher examples: a mole of hydrogen molecule, standard atomic weight of H is 1.00794, has the mass 2×1.00794 = ...hydrogen or oxygen atom? Does the article assume that everyone knows that hydrogen gas and oxygen gas are diatomic (meaning they have 2 atoms)? Should that no25 KB (4,281 words) - 01:20, 15 April 2008
- *Hydrogen and Fuel Cell Caucus3 KB (369 words) - 15:40, 22 March 2023
- ...from a smaller value (the energy used for the reaction). For example, when hydrogen burns:3 KB (432 words) - 22:07, 29 October 2020
- *[[Hydrodesulfurization|Naphtha Hydrotreater]] unit: Uses [[hydrogen]] to desulfurize the [[petroleum naphtha|naphtha]] fraction from the crude *[[Hydrodesulfurization|Distillate Hydrotreater]] unit: Uses hydrogen to desulfurize some of the other distilled fractions from the crude oil di13 KB (1,952 words) - 09:37, 6 March 2024
- ...s are not to be confused with [[steam reforming]] plants used to produce [[hydrogen]] and [[Ammonia production|ammonia]]. (b) Includes [[hydrogen]], methane, butenes, non-aromatic portion of [[pyrolysis gasoline]] and [[f9 KB (1,157 words) - 14:08, 2 February 2023
- ...ich spontaneously or catalyzed by [[superoxide dismutase]] dismutates to [[hydrogen peroxide]], as per the following reaction: Hydrogen peroxide and [[chlorine|chloride]] are subsequently converted by the enzyme7 KB (903 words) - 10:31, 10 June 2010
- |[[Hydrogen cyanide]]5 KB (597 words) - 08:35, 24 January 2011
- ...lt in products which no longer have the sour, foul odors of mercaptans and hydrogen sulfide. The liquid hydrocarbon disulfides may remain in the sweetened prod The feedstock entering the extractor must be free of any hydrogen sulfide (H<sub>2</sub>S) gas. Otherwise, any H<sub>2</sub>S entering the ex11 KB (1,728 words) - 09:37, 6 March 2024
- ...ay design of [[steam distillation]] columns for removing [[Gas|gaseous]] [[hydrogen sulphide]] from petroleum refinery wastewaters. Such columns are commonly r3 KB (400 words) - 10:27, 13 March 2024
- |[[Hydrogen |{{Nonmetal off |nonmetal color={{{nonmetal color}}} }} ]]12 KB (1,340 words) - 05:42, 6 March 2024
- |{{Periodic cell | eName=Hydrogen | eSym = H}}7 KB (880 words) - 05:43, 6 March 2024
- ...beam) are generated by the ions hitting the target, which has one or more hydrogen isotopes on its surface. <ref name=Sublette4.1>{{citation6 KB (938 words) - 21:00, 5 May 2010
- *[[Hydrodesulfurization|Naphtha Hydrotreater]] unit: Uses [[hydrogen]] to desulfurize the [[petroleum naphtha|naphtha]] fraction from the crude *[[Hydrodesulfurization|Distillate Hydrotreater]] unit: Uses hydrogen to desulfurize some of the other distilled fractions from the crude oil di14 KB (2,061 words) - 09:37, 6 March 2024
- ...terial from its binary partner. Most of the material is hydrogen. When the hydrogen reaches the surface of the white dwarf, it ignites, creating a nuclear expl6 KB (1,048 words) - 17:23, 26 July 2010
- :[[hydrogen]] (H<sub>2</sub>) : 1282.1 L·atm/mol ...with the example numerical values presented for oxygen, carbon dioxide and hydrogen and with their corresponding dimensional units.13 KB (2,084 words) - 05:21, 3 September 2013
- ...former or a gasifier to convert natural gas into [[carbon monoxide]] and [[hydrogen]], much as has been used in [[methanol]] and [[ammonia production]]. These3 KB (424 words) - 14:47, 26 April 2010
- <tr><td>[[Sulfide]]</td> <td>S<sup>2-</sup></td> <td> Evolves [[hydrogen sulphide]] (H<sub>2</sub>S) gas when sulfuric acid is added; Reduces Fe(III3 KB (610 words) - 09:37, 6 March 2024
- * [[Hydrogen]] atom: <math>\scriptstyle 1s\,\,\, ^2S_{\frac{1}{2}}</math>. Spin angular3 KB (467 words) - 01:09, 21 February 2010
- ...ssels operating at about 3,000 psig and 900 °F handling highly combustible hydrogen and hydrocarbons (as in a hydrocracking unit in a petroleum refinery), ther6 KB (939 words) - 16:52, 24 March 2024
- A simpler example is the combustion of [[hydrogen]] (H<sub>2</sub>) and oxygen, which is a commonly used reaction in [[rocket <center>'''hydrogen + oxygen → water vapor'''<br/>12 KB (1,825 words) - 17:36, 28 March 2021
- *[[Hydrogen bromide]] – HBr *[[Hydrogen chloride]] – HCl26 KB (3,686 words) - 08:29, 5 May 2024
- ...lt in products which no longer have the sour, foul odors of mercaptans and hydrogen sulfide. The liquid hydrocarbon disulfides may remain in the sweetened prod The feedstock entering the extractor must be free of any hydrogen sulfide (H<sub>2</sub>S) gas. Otherwise, any H<sub>2</sub>S entering the ex12 KB (1,901 words) - 09:37, 6 March 2024
- ..., in much the same way as when the sample consists of different compounds. Hydrogen chloride, for instance, would be seen as a mixture of the following isoto ...> 1 <td> [[Hydrogen|H]] <td width="5%" > [[Hydrogen/Atomic mass|{{:Hydrogen/Atomic mass}}]] <td width="15%"> <td align="left "> 38 <td> [[Stronti18 KB (2,483 words) - 09:47, 6 March 2024
- The gas chambers used [[Zyklon B]], a [[hydrogen cyanide]] preparation, rather than the [[carbon monoxide]] of other facilit3 KB (435 words) - 03:13, 27 March 2024
- ...omaticaceae (hydrogen sulfide as reducing equivalent donor), Chloroflexus (hydrogen as reducing equivalent donor) ..., Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)29 KB (4,037 words) - 02:19, 7 March 2024
- ...]s, a solid electrolyte connects the plates electrically while keeping the hydrogen and oxygen fuel gases separated.3 KB (486 words) - 04:36, 7 October 2009
- ...species by taking electrons from it, or by taking an electron as part of a hydrogen atom (H∙), or by adding oxygen, as the electrons of the oxidized species ...g agent reduces another species by supplying electrons to it, by supplying hydrogen (H∙) or by removing oxygen.31 KB (4,638 words) - 18:09, 29 October 2017
- {{r|Hydrogen bond}}4 KB (486 words) - 19:46, 11 January 2010
- ...examples of fermentation products are [[ethanol]], [[lactic acid]], and [[hydrogen]]. However, more exotic compounds can be produced by fermentation, such as ...acid is not the primary causes for the drop in pH, but rather ATP-derived hydrogen ions. Inorganic phosphate that increases during fatigue due to breakdown of8 KB (1,169 words) - 06:27, 9 June 2009
- ...operty}}}| cellWidth={{{cellWidth}}} | cellHeight={{{cellHeight}}} | eName=Hydrogen | colorscheme = {{{colorscheme}}}| eSym = H}}20 KB (1,639 words) - 05:43, 6 March 2024
- ...]] [[monomer]] consisting of an [[amino group]], a [[carboxyl group]], a [[hydrogen]] atom, and a residual group (commonly denoted as simply "R") [[covalent bo3 KB (443 words) - 21:24, 16 February 2010
- ...ticle of water; every particle of hydrogen is like every other particle of hydrogen, &c.''<ref>[http://web.lemoyne.edu/~giunta/dalton.html] excerpt from Dalton7 KB (1,170 words) - 08:30, 6 May 2022
- ...lecule]], can reach [[macroscopic]] sizes. The smallest molecule is the [[hydrogen]] molecule. The interatomic distance is 74 [[picometre]]s (0.74 [[Angstrom| ...ol]] (CH<sub>3</sub>CH<sub>2</sub>OH) is always composed of [[carbon]], [[hydrogen]], and [[oxygen]] in a 2:6:1 ratio, i.e., its empirical formula is C<sub>2<11 KB (1,558 words) - 21:27, 10 November 2020
- |{{Cel | cw={{{cellWidth}}} | ch={{{cellHeight}}} | nam=Hydrogen | cs = {{{colorscheme}}}| eSym = H}}13 KB (1,413 words) - 05:43, 6 March 2024
- ...hylene and methane, and found that methane contained exactly twice as much hydrogen as ethylene. Why this mathematical simplicity?....Again Dalton made models3 KB (538 words) - 21:11, 4 June 2009
- ...her; such as the synthesis of [[water]] (H<sub>2</sub>O) from two gases: [[hydrogen]] (H<sub>2</sub>) and [[oxygen]] (O<sub>2</sub>). Some chemical interaction ...nough to be a gas. Note that in each case, energy is required to break the hydrogen bonds, as well as to further separate the molecules from each other.23 KB (3,309 words) - 09:41, 6 March 2024
- The two hydrogen atoms, labeled ''A'' and ''B'', each have one electron (a red arrow in the With regard to the form of the MOs the following: Because both hydrogen atoms are identical, the molecule has reflection symmetry with respect to a8 KB (1,408 words) - 09:47, 24 April 2010
- ...in generation of atomic energy, or energy to mass, as in the generation of hydrogen atoms from the energy released by the Big Bang that originated our universe4 KB (613 words) - 20:17, 5 June 2011
- ...otope labeling|isotopic]] [[desymmetrisation]] on the substrate (replacing hydrogen by [[isotope labeling|deuterium]]) it can be demonstrated that the reaction ...-H bond (105 compared to 148 pm) and weaker (338 compared to 299 KJ/mole). Hydrogen is more [[electronegative]] than silicon hence the naming convention of sil9 KB (1,169 words) - 02:10, 27 October 2013
- |{{Rpc |{{{1|}}}|{{{2|}}}|{{{3|}}}|{{{4|}}}|{{{5|}}}|{{{6|}}}| eName=Hydrogen | colorscheme = {{{colorscheme}}}| eSym = H}}16 KB (965 words) - 05:43, 6 March 2024
- ...a different form with a different set of properties, as, for example, when hydrogen atoms and oxygen atoms transform into liquid water, or when liquid water tr ...in generation of atomic energy, or energy to mass, as in the generation of hydrogen atoms from the energy released by the Big Bang that originated our universe14 KB (2,271 words) - 17:17, 9 October 2013
- ...synthesis of [[water (molecule)|water]] (H<sub>2</sub>O) from two gases: [[hydrogen]] (H<sub>2</sub>) and [[oxygen]] (O<sub>2</sub>). Some chemical interaction ...nough to be a gas. Note that in each case, energy is required to break the hydrogen bonds, as well as to to further separate the molecules from each other.22 KB (3,142 words) - 09:01, 4 May 2024
- *Hydrogen fuel generator using water as feedstock, but perhaps a [[Very High Temperat ...from closed loops? I honestly don't know. Lots of people seem to think of hydrogen-fueled vehicles as using renewable fuel. [[User:Howard C. Berkowitz|Howard18 KB (2,925 words) - 22:08, 18 July 2010
- ...ired to replace the hydrogen atom in HF (hydrogen fluoride) by a different hydrogen atom; and for others as well. The author, H. F. Schaefer, argued that good14 KB (2,214 words) - 16:43, 14 July 2009
- ...ating each of the three anions, those formed with dihydrogen phosphate and hydrogen phosphate decompose upon heating, producing a metal [[trimetaphosphate]],M<4 KB (532 words) - 23:27, 20 February 2010
- ...[[Maurice R. Pierce]] in command. The airship was also switched over from hydrogen to helium gas, which reduced payload but improved safety.3 KB (538 words) - 10:06, 10 February 2023
- Hot air craft produce much less lift per unit volume than Helium or Hydrogen filled craft (about 30% depending on air conditions)4 KB (586 words) - 06:17, 12 September 2013
- ...uld be transferred into particle momentum (Xray scattering on nearly free hydrogen atoms). The present article is not in agreement with my memory, so who is3 KB (478 words) - 17:02, 5 March 2024
- ...s|synthesis]] or by other means) of chemical compounds of [[carbon]] and [[hydrogen]], which may contain any number of other elements, such as [[nitrogen]], [[ ...s simplicity without introducing ambiguity, whilst representing carbon and hydrogen by implication. The disadvantage which arises from the fact that structural21 KB (3,106 words) - 09:10, 5 May 2024
- ....gov/pmc/articles/PMC1063337/?tool=pmcentrez The Structure of Proteins Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain]. Proc Natl Acad Sci ...mystery was the question of how the nucleotides were bonded together, via hydrogen bonds or salt linkages from negatively charged phosphate groups.8 KB (1,287 words) - 10:14, 27 December 2020
- ...cular complexes that mediate electron flow from water to carbohydratess or hydrogen gas are present the electron-transport chain found in the thylakoid membran9 KB (1,262 words) - 16:26, 23 September 2013
- ...ecular complexes that mediate electron flow from water to carbohydrates or hydrogen gas are present the electron-transport chain found in the thylakoid membran9 KB (1,262 words) - 09:17, 11 October 2013
- |[[Hydrogen]]|| align="center"|H<sub>2</sub>|| align="center"|0.000050|| align="center" ...temperature as well as a [[catalyst]] to combine atmospheric nitrogen and hydrogen to form ammonia which can be used directly as fertilizer or processed furth12 KB (1,867 words) - 08:51, 30 June 2023
- ...of the airship to use [[hydrogen]] as the lift gas, despite the fact that hydrogen, unlike helium, is extremely flammable.<ref name="botting"> Botting 2001, p ...zeppelins, so the switch from helium to hydrogen did not cause much alarm. Hydrogen also gave the craft about 8% more lift capacity.26 KB (4,090 words) - 07:31, 20 April 2024
- ...is that liquid water exists as a continuously rearranging [[hydrogen bond|hydrogen-bonded]] network with motions on the picosecond (10<sup>−12</sup> s) ...luminescence, entanglement described by a new quantum theory, formation of hydrogen peroxide, clathrate formation, etc. without any mechanism singularly standi11 KB (1,596 words) - 08:22, 31 May 2010
- [[carbon dioxide]] (O=C=O), [[hydrogen cyanide]] (HC≡N), and [[carbonyl sulfide]] (O=C=S). Examples of larger l5 KB (704 words) - 05:59, 26 September 2007
- '''Water''' is a [[chemical compound]] composed of [[hydrogen]] and [[oxygen]] and has the [[chemical formula]] (H<sub>2</sub>O or HOH). ...nd outermost) shell, which has a natural capacity of eight electrons. Two hydrogen atoms can thus share electrons with one oxygen atom, filling their respecti24 KB (3,756 words) - 01:56, 29 April 2021
- A combustion analysis for a few typical fuel cases (carbon, hydrogen, sulfur, coal, oil and gas) when the fuel reacts with air at stoichiometric ...that the enthalpy value for basic combustion elements such as carbon (C), hydrogen (H<sub>2</sub>), sulfur (S), oxygen (O<sub>2</sub>) and nitrogen (N<sub>2</52 KB (8,113 words) - 10:19, 30 May 2009
- ...some of the energy release in the combustion is derived from combustion of hydrogen rather than carbon which would affect the amount of carbon dioxide emission8 KB (1,316 words) - 15:37, 2 October 2013
- ...emistry]] of carbon allows it to bond with many other elements, especially hydrogen, oxygen, nitrogen and phosphorus, and, even to form carbon-to-carbon bonds.4 KB (625 words) - 06:28, 6 March 2024
- ...content of the blood and body tissues, and characterized by an increase in hydrogen ion concentration (decrease in pH). (Dorland, 27th ed)."<ref>{{MeSH}}</ref>4 KB (527 words) - 06:23, 5 February 2011
- ...he third most abundant [[Chemical element|element]] in the universe (after hydrogen and helium) and the most abundant element of the [[lithosphere]] (about 47% ...t is electron transport from cathode to anode and the formation of gaseous hydrogen at the cathode and gaseous oxygen at the anode. This process, which require12 KB (1,791 words) - 05:43, 6 March 2024
- ...atson and Crick soon produced their [[double helix]] model of DNA with the hydrogen bonds at the core of the helix providing a way to unzip the two complementa ...a DNA molecule. Just pull apart the two phosphate backbones, each with its hydrogen bonded A, T, G, and C components. Each strand could then be used as a templ13 KB (2,038 words) - 06:56, 9 June 2009
- Given that non-destructive techniques like [[Hydrogen|<sup>1</sup>H]] [[Nuclear magnetic resonance|MR]] [[Magnetic resonance imag4 KB (579 words) - 04:54, 21 March 2024
- ..., Baltazar de Castro, Frank T. Robb and Wilfred R. Hagen (2000) Enzymes of hydrogen metabolism in Pyrococcus furiosus. European Journal of Biochemistry, 267, 64 KB (610 words) - 04:31, 22 November 2023
- ...ranules found in the cytoplasm of this organism is due to the oxidation of hydrogen sulfide in to sulfur. ''Thiomargarita namibiensis'' is a non-motile organis5 KB (690 words) - 05:33, 28 November 2013
- ...(see [[Hydrogen-like atom#Quantum numbers of hydrogen-like wave functions|hydrogen-like atom]] for the meaning of ''p'' and ''l'' ) can be found as linear co15 KB (2,490 words) - 12:23, 19 April 2009
- :"The [[Haber process]], which is the production of ammonia by combining hydrogen and [[nitrogen]], was first patented by [[Fritz Haber]] in 1908. In 1910, [ ...t color=green>The Haber process for the production of ammonia by combining hydrogen and [[nitrogen]] was first patented by a chemist, [[Fritz Haber]], in 1908.10 KB (1,536 words) - 15:30, 2 October 2013
- ...d shaped bacteria that contain [[peritrichous]] [[flagella]] and produce [[hydrogen sulphide]]. Important molecules that they produce once inside their hosts a ...hey contained three membrane associated [[enzymes]] that helped break down hydrogen.20 KB (3,123 words) - 09:37, 6 March 2024
- Ethan, Don't forget to link somewhere to [[hydrogen-like atom]], where the SE is solved. Cheers, --[[User:Paul Wormer|Paul Worm4 KB (633 words) - 01:57, 18 December 2010
- ...Centauri is a very active variable star. As a star of low mass it converts hydrogen to helium very slowly compared to our own star. Sol. This slow conversion c5 KB (794 words) - 18:54, 20 December 2007
- ...bers used carbon monoxide, for large scale use, they used a preparation of hydrogen cyanide, adsorbed onto inert material forming a pesticide product called Zy ...ydrogen cyanide and cyanogen chloride are quite lethal in confined spaces; hydrogen cyanide was the principal lethal agent used in Nazi genocide. It is quite d14 KB (2,220 words) - 07:28, 18 March 2024
- * ''Hydrogen (molecular mass of 2.016 g · mol<sup>–1</sup>)'' : '''''R'''''<sub>s</s5 KB (821 words) - 16:14, 14 October 2013
- ...me known as [[Fusion device|thermonuclear]] weapons (also referred to as [[hydrogen bomb]]s or [[H-bomb]]s). Thermonuclear weapons are based on the energy rele ...astle Bravo H-Bomb, 1954.jpg|left|230px|Fireball of Castle Bravo 1 megaton hydrogen bomb test at Bikini Atoll, March 1, 1954}}19 KB (2,853 words) - 09:20, 22 April 2024
- ...an and deodorize wounds and [[ulcer]]s. More common 1% or 2% solutions of hydrogen peroxide have been used in household first aid for scrapes, etc. However,9 KB (1,302 words) - 19:31, 11 February 2010
- ...r structure]] in the center of the Galaxy. With improved radio telescopes, hydrogen gas could also be traced in other galaxies. In the 1970s it was discovered .... However, the supply of star-forming material is finite; as stars convert hydrogen into heavier elements, fewer stars will form.17 KB (2,688 words) - 22:56, 16 January 2021
- Although organic molecules contain a variety of atomic elements (especially hydrogen, oxygen, nitrogen, phosphorus, and sulfur), they always have a predominant ...icular covalent bonding capacity of carbon thus enables it to combine with hydrogen, oxygen, nitrogen, and itself in multi-varied ways that generate small carb19 KB (2,914 words) - 05:42, 6 March 2024
- |[[Hydrogen |{{ {{#ifeq:{{{elSym}}}|H33 KB (3,249 words) - 05:42, 6 March 2024
- ...d hydrocarbons]] ([[Hydrocarbon|alkenes]]) by reacting them with gaseous [[hydrogen]] (H<sub>2</sub>) to produce liquid [[Hydrocarbon|saturated hydrocarbons]] *[[Claus process]]: Convering gaseous [[hydrogen sulphide]] into elemental [[sulphur]]21 KB (3,174 words) - 07:31, 20 April 2024
- ...person, an FM radio signal, air) or have likely heard about frequently (a hydrogen atom, the speed of light).4 KB (629 words) - 16:39, 8 April 2022
- The Bohr radius was the distance of an electron from the nucleus of a hydrogen atom predicted by the Bohr theory of the atom, which required an integer nu ...dius is the distance of maximum likelihood for finding the electron in the hydrogen atom in its ground state. <ref name=Demtröder>13 KB (1,945 words) - 19:19, 1 June 2022
- ...ster B. Kay (1953). "Phase-Equilibrium Properties of System Carbon Dioxide-Hydrogen Sulfide." ''Industrial Engineering & Chemistry'' '''45'''(3): 618-624 '''<n ...ster B. Kay (1953). "Phase-Equilibrium Properties of System Carbon Dioxide-Hydrogen Sulfide." ''Industrial Engineering & Chemistry'' '''45'''(3): 618-62425 KB (3,806 words) - 02:27, 19 May 2011
- Cobalt(II) oxide rapidly decomposes hydrogen peroxide and oxidizes the drying of unsaturated oils in an exothermic react5 KB (712 words) - 21:11, 22 February 2009
- <tr><td> H <td> [[Hydrogen]] <td align="right"> 18 KB (1,135 words) - 09:15, 6 March 2024
- ...N<sub>2</sub>) and a [[hydrogen]] atom with a [[fluorine]] atom forming [[hydrogen fluoride]] (HF). As an example of larger complexes, [[carbon]] (C) and [ ...e very common in [[organic chemistry]]. They occur between, for instance, hydrogen, carbon, nitrogen, and oxygen atoms and give rise to stable, recognizable,37 KB (5,836 words) - 05:36, 6 March 2024
- [[hydrohalic acid|Hydrohalic acids]], diatomic compounds of one hydrogen atom and one halogen atom, are particularly strong acids owing to high halo6 KB (813 words) - 06:00, 6 March 2024
- ...tor (quantum)|harmonic oscillator]]s). A more complex system even is the [[Hydrogen |H<sub>2</sub>]] molecule where only approximations to the solutions can be * The [[hydrogen atom]] or [[hydrogen-like atom]]17 KB (2,678 words) - 10:12, 9 May 2011
- ...n important fuel source and a major feedstock for producing [[ammonia]], [[hydrogen]], [[petrochemicals]] and [[fertilizer]]s. *[[Acid gas]]es: [[carbon dioxide]] (CO<sub>2</sub>), [[hydrogen sulphide]] (H<sub>2</sub>S) and [[mercaptan]]s such as [[methanethiol]] (CH26 KB (3,931 words) - 09:37, 6 March 2024
- ...n important fuel source and a major feedstock for producing [[ammonia]], [[hydrogen]], [[petrochemicals]] and [[fertilizer]]s. *[[Acid gas]]es: [[carbon dioxide]] (CO<sub>2</sub>), [[hydrogen sulphide]] (H<sub>2</sub>S) and [[mercaptan]]s such as [[methanethiol]] (CH26 KB (3,927 words) - 09:37, 6 March 2024
- * [[hydrogen]] can ignite itself in a bad leak ...aham, glad to see you back. I have no problem with your first two items ([[hydrogen]] and [[steam]]). Go ahead and add your content about them.10 KB (1,579 words) - 22:08, 29 April 2011
- ...are the same in every respect. In general, coal consists of [[carbon]], [[hydrogen]], [[oxygen]], [[nitrogen]], [[sulphur]] and [[mineral]] matter (including ...oisture and ash. It is composed primarily of carbon with lesser amounts of hydrogen, nitrogen and sulfur.20 KB (3,084 words) - 09:16, 6 March 2024
- ...]] and 4% [[atoms]]. Thus the density of atoms is on the order of a single hydrogen [[nucleus]] (or atom) for every four cubic meters of volume. The exact natu ...rst stars, the chemical composition of the Universe consisted primarily of hydrogen (75% of total mass), with a lesser amount of helium-4 (4He) (24% of total m10 KB (1,592 words) - 08:35, 15 November 2007
- ...r, and the removal of gases such as [[ammonia]], [[carbon dioxide]] and [[hydrogen sulphide]] from water. ...opylene glycol dimethyl ether) to absorb and remove [[acid gas]]es such as hydrogen sulfide and/or carbon dioxide from gas streams. The solvent is then strippe11 KB (1,664 words) - 09:37, 6 March 2024
- ...ase of medical MRI, the most commonly used nucleus is the nucleus of the [[hydrogen]] [[atom]]. Most biomedical MR images are essentially plots showing the dis Atoms with an odd number of [[nucleon]]s (protons and neutrons), such as [[hydrogen]] and [[carbon]]-13 (but not carbon-12!) possess an intrinsic degree of fre10 KB (1,408 words) - 04:54, 21 March 2024
- ...proportions. For instance, 1 liter of oxygen gas combines with 2 liters of hydrogen gas to form 2 liter of gaseous water. Especially Gay-Lussac's law was of gr ...ad assumed earlier that water is formed from a molecule each of oxygen and hydrogen, rejects Avogadro's and Gay-Lussac's laws.19 KB (2,947 words) - 20:20, 27 December 2020
- ...-561 has an extremely low abundance of metals, or any element heavier than hydrogen or helium, and is very old; Weiss calculates an age of roughly 10 billion y ...believed to be far too small and irradiated to hold onto its primordial [[Hydrogen]] and [[Helium]] envelope. However, the composition of the planet varies gr13 KB (1,765 words) - 11:12, 23 July 2022
- * Silva PJ ''et al.'' (2000) Enzymes of hydrogen metabolism in Pyrococcus furiosus. [http://dx.doi.org/10.1046/j.1432-1327.25 KB (616 words) - 05:04, 1 February 2008
- ...it in that it was the first rigid airship to use [[helium]] rather than [[hydrogen]]. A similar precaution might have prevented the [[Hindenburg disaster]] tw ...rship contained [[helium]], which does not react chemically with air. If [[hydrogen]] had been used, the ship probably would have burned - as the ''[[LZ 129 Hi10 KB (1,528 words) - 09:44, 5 August 2023
- ...). The element hydrogen, for example, is a substance that consists only of hydrogen atoms, the element oxygen consists only of oxygen atoms, and so on.</ref> T ...g the 94 naturally occurring chemical elements, the atoms of the element [[hydrogen]], Z=1, have the fewest number of protons, and those of the element [[pluto33 KB (4,730 words) - 09:16, 6 March 2024
- ...original research in any other way are not allowed, such as a diagram of a hydrogen atom showing extra particles in the nucleus as theorized by the uploader.6 KB (885 words) - 13:57, 12 January 2008
- ...The enzyme [[catalase]], found exclusively in peroxisomes, converts the [[hydrogen peroxide]] into [[water]] and [[oxygen]].5 KB (813 words) - 16:55, 8 May 2010
- ... sulfur or sul- fides such as hydrogen sulfide and ... the earth and that a primordial lithoautotrophy, hydrogen, phylogeny, community structure, repository14 KB (2,123 words) - 05:02, 30 October 2011
- ...a level). The primary advantage of helium is that it non-flammable whereas hydrogen burns readily in air. ...filled with hydrogen. Nonetheless, some small experimental ships still use hydrogen today.23 KB (3,524 words) - 07:41, 12 April 2014
- ...truly perfect, not even in interstellar space, where there are still a few hydrogen atoms per cubic centimetre.<ref name=Tadokoro>{{cite journal5 KB (754 words) - 15:12, 4 August 2011
- ...of [[pneumonia]]. A mixture of five-percent acetic acid and three-percent hydrogen peroxide is commonly used.18 KB (2,906 words) - 10:10, 28 February 2024
- ...><ref>Elsaesser T (2009) Ultrafast memory loss and relaxation processes in hydrogen-bonded systems ''Biol Chem'' 390:1125-32 (Review) PMID 19663683</ref><ref>K ...ared with pure water. Rey claimed that this suggested that the networks of hydrogen bonds in homeopathic dilutions were different. <ref>Rey L (2003)Thermolumin18 KB (2,650 words) - 03:19, 25 June 2019
- ...ng point of the compound. Simple [[carboxylic acid]]s dimerize by forming hydrogen bonds between molecules. A minor factor affecting boiling points is the sh15 KB (2,372 words) - 00:31, 28 October 2013
- ...ng point of the compound. Simple [[carboxylic acid]]s dimerize by forming hydrogen bonds between molecules. A minor factor affecting boiling points is the sh15 KB (2,373 words) - 19:13, 5 August 2018
- ...n]] and/or [[air]]) to produce a mixture of [[carbon monoxide]] (CO) and [[hydrogen]] (H<sub>2</sub>) commonly called ''syngas'' (a contraction of ''synthesis ...syngas may be directly burned as a fuel, converted into methyl alcohol or hydrogen, subjected to [[methanation]] to produce [[synthetic natural gas]] (SNG),<r17 KB (2,437 words) - 02:47, 21 March 2024
- ...n]] towers (called [[sour water strippers]]) to strip virtually all of the hydrogen sulfide and somewhat less of the ammonia from aggregated sour waters.<ref n12 KB (1,965 words) - 09:37, 6 March 2024
- Further examples: a mole of [[hydrogen]] molecule, standard atomic weight of H is 1.00794, has the mass 2×1.5 KB (914 words) - 14:09, 2 February 2023
- Diaphragm compressors are used for hydrogen and compressed natural gas ([[Compressed natural gas|CNG]]) as well as in a ...[[hydrogen]] gas to 6,000 psi (41 MPa) for use in a prototype [[compressed hydrogen]] and [[compressed natural gas]] (CNG) fueling station built in downtown [[17 KB (2,493 words) - 19:22, 17 February 2010
- ...itively charged particles, [[proton]]s and—with the exception of the hydrogen nucleus—one or more electrically neutral particles, [[neutron]]s. The5 KB (827 words) - 17:02, 22 March 2024
- ...mpacting the neutron economy. Steel does not suffer the rapid oxidation or hydrogen generation at high temperature that zirconium alloys do. If there is a clad12 KB (1,883 words) - 10:13, 10 May 2024
- | Proximal convulated tubule || 40% || Carbonic anhydrase<br>[[Sodium-hydrogen antiporter]] ([[Ion pump]]) || Carbonic anhydrase inhibitors6 KB (806 words) - 10:40, 24 July 2008
- |hazard=Reacts violently with water and acids to release explosive hydrogen,6 KB (899 words) - 11:12, 20 November 2022
- ...densities of 1.3 and 0.69 g/cc, respectively. Composed mainly of the gases hydrogen and helium. ...methane, and ammonia, with a small rocky core and an atmosphere of mostly hydrogen and helium.12 KB (1,829 words) - 10:07, 10 January 2021
- ...greater amounts of energy by joining the nuclei of lighter elements (e.g., hydrogen isotopes) into a heavier one (e.g.,helium). | title = Dark Sun: the Making of the Hydrogen Bomb20 KB (3,072 words) - 10:33, 18 March 2024
- It is composed largely of [[hydrogen]] and [[helium]]. Jupiter's strong internal heat creates a number of semi-6 KB (921 words) - 08:26, 10 January 2021
- ...hich have the nitrogenase [[enzyme]] that combines gaseous nitrogen with [[hydrogen]] to produce [[ammonia]], which is then further converted by the bacteria t ...reat pressure at 600°C, and using a [[catalyst]], atmospheric nitrogen and hydrogen (usually derived from [[natural gas]] or [[petroleum]]) can be combined to21 KB (3,189 words) - 15:35, 3 September 2010
- ...ed by the hydroformylation reaction of [[alkene]]s, carbon monoxide, and [[hydrogen]]. Hydroformylation is coupled to Shell Chemical's Higher Olefin Process (S ...ammatory responses in the body (the other two being [[nitric oxide]] and [[hydrogen sulphide]]), carbon monoxide has received a considerable attention as a bio17 KB (2,453 words) - 09:37, 6 March 2024
- ...ebster B. Kay |title=Phase-Equilibrium Properties of System Carbon Dioxide-Hydrogen Sulfide |journal=Ind. Eng. Chem. |volume=45 |issue= 3 |date=March, 1953 |pa ...ebster B. Kay |title=Phase-Equilibrium Properties of System Carbon Dioxide-Hydrogen Sulfide |journal=Ind. Eng. Chem. |volume=45 |issue= 3 |date=March, 1953 |pa14 KB (2,296 words) - 18:04, 17 May 2011
- ...of the planet plunged into a long, dark winter. Uranus is a ball of mostly hydrogen and helium. Absorption of red light by methane in the atmosphere gives the5 KB (895 words) - 23:55, 29 November 2011
- The reaction is first order in H<sub>2</sub>, as the hydrogen concentration is raised to the power of 1; it is second order in NO, accord7 KB (1,127 words) - 05:54, 31 October 2011
- ...iscrete mathematics|discrete]] (e.g., the energy of an electron bound to a hydrogen atom). ...ial energy]]. The article on the [[Stark effect]] treats the example of a hydrogen atom in an external electric field.37 KB (5,578 words) - 04:54, 21 March 2024
- ...ydrogen'', which is highly unstable. Personally I would not call elemental hydrogen a "substance".--[[User:Paul Wormer|Paul Wormer]] 05:00, 12 June 2009 (UTC) ...ckel) exists in atomic form. Now which of these substances is the element hydrogen?57 KB (9,089 words) - 08:34, 6 March 2024
- ...of his role as an advocate and conceptual designer of the [[fusion device|hydrogen bomb]], his outspoken defense of an unassailable nuclear arsenal, and suppo ...Teller, Zeitschrift für Physik, ''Über das Wasserstoffmolekülion'' [On the hydrogen molecule ion], vol. '''61''', pp. 458–480 (1930)</ref>28 KB (4,424 words) - 07:32, 20 April 2024
- ...the upper layers of the atmosphere. Neptune is comprised predominantly of hydrogen and helium. However, its methane component reaches the upper atmosphere ab6 KB (904 words) - 19:12, 9 January 2021
- == Article of the week: Hydrogen bond == I just changed the article status of ''Hydrogen bond'' from a "3" to a "1". It was nominated for article of the week (front20 KB (3,069 words) - 09:35, 11 March 2009
- ...densest solids and the lightest gas, which is about 250,000 for osmium vs. hydrogen at atmospheric pressure and room temperature. The number of hydrogen atoms in 1 gram, or the number of carbon-12 atoms in 12 grams45 KB (6,572 words) - 12:36, 9 March 2024
- ...hylene and methane, and found that methane contained exactly twice as much hydrogen as ethylene. Why this mathematical simplicity? <ref name=jaffe76>Jaffe B. ( ...tomic weights of different elements — relative to the unit weight of hydrogen.26 KB (4,140 words) - 06:36, 6 March 2024
- <td rowspan="1"> For the fine structure of the hydrogen spectrum ...wspan="1"> For elementary particle physics, discovery of resonance states, hydrogen bubble chamber and data analysis30 KB (3,679 words) - 09:07, 12 October 2013
- ...The unpaired electrons in the three 3''p'' orbitals bind with those in the hydrogen 1''s'' orbitals to form electron pairs of opposite [[spin]]. The electron p ...tructures with the phosphorus or arsenic atom in the center bound to three hydrogen atoms and one lone electron pair. Both are colorless, ill-smelling, toxic19 KB (2,983 words) - 05:36, 6 March 2024
- ...The unpaired electrons in the three 3''p'' orbitals bind with those in the hydrogen 1''s'' orbitals to form electron pairs of opposite [[spin]]. The electron p ...tructures with the phosphorus or arsenic atom in the center bound to three hydrogen atoms and one lone electron pair. Both are colorless, ill-smelling, toxic19 KB (2,982 words) - 05:36, 6 March 2024
- ...rocesses.</ref> and fuels for the transportation sector.<ref>Generation of hydrogen for fuel is possible with a [https://www.gen-4.org/gif/jcms/c_9362/vhtr Ver ...sing helium gas as a coolant, might provide process heat for production of hydrogen fuel from water.<ref>https://www.gen-4.org/gif/jcms/c_9362/vhtr</ref>14 KB (2,250 words) - 17:04, 11 October 2021
- ...star burns [[hydrogen]] in a process that synthesises [[helium]]. When the hydrogen is consumed, the helium is then burned and that in turn synthesises carbon.14 KB (2,338 words) - 10:18, 23 November 2011
- === Bohr's model of the Hydrogen Atom ===18 KB (2,789 words) - 20:34, 27 October 2020
- ...nosine triphosphate + water → Adenosine diphosphate + phosphate ion + hydrogen ion ...the biosynthesis of [[fatty acid]]s. Reducing power is usually supplied as hydrogen equivalents carried by [[NADPH]]. Organisms can be classified according to14 KB (2,059 words) - 12:47, 6 September 2013
- ...nosine triphosphate + water → Adenosine diphosphate + phosphate ion + hydrogen ion ...the biosynthesis of [[fatty acid]]s. Reducing power is usually supplied as hydrogen equivalents carried by [[NADPH]]. Organisms can be classified according to14 KB (2,063 words) - 12:41, 6 September 2013
- ...and the [[oxygen|O]] are bound by a double bond, the [[Nitrogen|N]] and [[Hydrogen|H]] with a single bond, the combination is a [[carbonyl group]]). The amino ...here all bonds between the carbon atoms have been completely saturated (by hydrogen). The simplest hydrocarbon is [[methane]] (CH<sub>4</sub>); methane is a [[36 KB (5,455 words) - 11:49, 6 September 2013
- ...and the [[oxygen|O]] are bound by a double bond, the [[Nitrogen|N]] and [[Hydrogen|H]] with a single bond, the combination is a [[carbonyl group]]). The amino ...here all bonds between the carbon atoms have been completely saturated (by hydrogen). The simplest hydrocarbon is [[methane]] (CH<sub>4</sub>); methane is a [[36 KB (5,455 words) - 08:57, 12 September 2013
- ...diation. An example is the conversion of water into [[hydrogen]] gas and [[hydrogen peroxide]]. ...the formation of organic waste which contains elements other than carbon, hydrogen, nitrogen and oxygen. Such an organic waste can be burned without forming a31 KB (4,881 words) - 12:55, 15 March 2024
- ...calculate neutron cross-sectional densities to help in the design of the [[hydrogen bomb]]<ref>{{cite web | title=Classical Super / Runaway Super | year=Unknow7 KB (1,016 words) - 08:49, 22 April 2024
- ...drogen.energy.gov/pdfs/progress05/v_e_1_shimko.pdf US Department of Energy Hydrogen Program]</ref> and other low-temperature processes. ...s amounts of [[acid gas]]es such as [[carbon dioxide]] (CO<sub>2</sub>), [[hydrogen sulphide]] (H<sub>2</sub>S) and [[mercaptan]]s such as [[methanethiol]] (CH16 KB (2,411 words) - 09:37, 6 March 2024
- ...n pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo.6 KB (902 words) - 03:35, 15 April 2009
- ...The enzyme [[catalase]], found exclusively in peroxisomes, converts the [[hydrogen peroxide]] into [[water]] and [[oxygen]]. Acyl-CoA entry into the peroxisso7 KB (1,029 words) - 16:31, 25 March 2010
- ...e. Perhaps it would help to include a small table comparing pH values with hydrogen ion concentrations, over the ranges of pH discussed in the article.7 KB (1,196 words) - 15:04, 2 October 2013
- ...about 2.4 m/s. The landing rockets used an 18 nozzle design to spread the hydrogen and nitrogen exhaust over a wide area. It was determined that this would li6 KB (935 words) - 07:34, 9 June 2009
- ...he plan is to solve it, for example in connection with a discussion of the hydrogen atom).7 KB (1,123 words) - 14:02, 14 November 2007
