Archive:Article of the Week
The Article of the Week is an article chosen by vote among Citizens as exemplifying various qualities we like to see in a Citizendium article; see our article standards.
Current Nominees
The next article (or draft) of the week will be the article with the most votes at 1 AM UTC on Thursday, 11 June 2009. I did the honors this time. Milton Beychok 07:10, 5 June 2009 (UTC)
| Nominated article | Supporters | Specialist supporters | Score | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Kop needed to change the formatting in Ancient Celtic music so that {{Featured Article Candidate}} works. |
Alexander Wiebel 12:29, 19 May 2009 (UTC); Daniel Mietchen 17:24, 4 June 2009 (UTC); Meg Ireland 14:36, 5 June 2009 (UTC) |
3 | ||||||||||||||||||||||||||||||||||||
Bifrenaria is a genus of orchids containing twenty South American species. some of which rank among the favorites of orchid growers because of their abundant showy flowers, which are large and very fleshy and at first glance seem to be artificial flowers made of wax. There are no known uses for them other than ornamental. Despite having few species, the genus can be split in two clearly distinct groups:[1] one of highly robust plants with large flowers, that encompass the first species to be classified under the genus Bifrenaria; other of more delicate plants with smaller flowers occasionally denominated Stenocoryne or Adipe. There are yet two other species that normally are classified as Bifrenaria but which molecular analysis indicate to belong to different orchid groups. They are Bifrenaria grandis, endemic of Bolívia, which many taxonomists denominate Lacaena grandis,[2] and Bifrenaria steyermarkii, inhabitant of northern Amazon Forest,[3] which does not have an alternative classification as yet. Distribution (CC) Photo: Dalton Holland Baptista Bifrenaria inodora Despite described with the name of B. inodora, this large species is one of the most fragrant Bifrenaria. It has two main varieties, the one on the photo was also described as B. fuerstenbergiana and has this varietal name, the other one is greener and has a yellow labellum. Bifrenaria exist from the north of South America, one species reaching Trinidad, until Rio Grande do Sul, the farther south State in Brazil, however they are split in two isolated areas:[4] Amazon Forest and Atlantic Forest of Brazil. The later, where seventeen species are present, may be considered their recent center of distribution. The mountain area of Rio de Janeiro State and Espírito Santo is particularly rich with fifteen species registered. Serra dos Órgãos mountains area, in Rio, is reported as habitat of fourteen Bifrenaria species,[5] however, some of these species are considered synonyms today,[6] being eleven a more realistic number of species existing in the said area. The species with large flowers are more common on Region Southeast of Brazil, however, they inhabit from the sunnier areas of the seashore to rocky mountain areas of Minas Gerais e Bahia States, from almost sea level up to 2,000 meters of altitude, some species reaching Rio Grande do Sul state.[7] No species of large flowers exist in Amazon Forest. Some species grow directly attached to the famous Sugarloaf Mountain in Rio de Janeiro which can be observed by the commuters in the cable car. The recent centers of irradiation of this group are the seashore close to Serra do Mar chain of mountains, and the high chains of mountains of Minas Gerais.[8] The most common species in this group, spread from Rio Grande do Sul to Bahia, is B. harrisoniae.[9] The smaller species of Bifrenaria, which some taxonomists classify under the genus Adipe, are more common on less sunny areas and can be found between 300 and about 1,600 meters of altitude.[5] Six species are native in Serra do Mar Chain of mountains and its arms, place considered the center of distribution of the small species. Only three small species inhabit Amazon, B. longicornis, which is more common at low altitudes;[10] Bifrenaria venezuelana, up to 1,450 meter of altitude, and B. steyermarkii from even higher altitudes, in Roraima State, in Brazil, and nearby areas in Venezuela and Surinam.[11] The most common species is B. aureofulva,[12] however, because the geographic characteristic of its territory, without obstacles, B. longicornis is the species spread throughout the largest area, reaching Colombia, Venezuela, Peru, Surinam, Guyanas, Trinidad and all Amazonic area in Brazil.[13] Two species seem to be endemic in highly restricted areas: B. silvana. discovered in 1987 at Serra da Ouricana mountains, nearby Itororó, in Bahia which belongs to Adipe group;[14] and B. verboonenii, discovered in September 1995 on Serra do Cipó mountains, close to Diamantina, Minas Gerais, of the large Bifrenara group.[15] Bifrenaria species inhabit three different environments. The large species generally live in well illuminated areas, occasionally epiphyte on trees of sparse foliage, more often as lithophytes, in campos rupestres, montane rocky areas that exist mostly in Rio and Minas Gerais States of Brazil, or over rocks in jungle's clearances. B. tyrianthina is exclusively lithophyte,[16] B. tetragona and B. wittigii hardly ever do. B. atropurpurea is the only species found living terrestrially, but in rare occasions. The large species always show .[10] The small species from Southeast Brazil live in cloud montane forests, where the appear in much darker places than the large species. Within this sort of forests the temperature presents noticeable difference between day and night and also through the seasons. These also are plants of caespitous growth, almost all epiphytes, despite there is at least one record of Bifrenaria aureofulva living lithophylically in Chapada Diamantina, Bahia.[17] The species from Amazon inhabit tropical lowland forests and equatorial forests. Bifrenaria longicornis is mostly found in flood areas along the igapós and igarapés (seasonal flood streams and small rivers of Amazon), and occasionally in open fields where the humidity is high and temperature constant through the year, normally in well illuminated places, although not under direct sunlight.[18] B. venezuelana inhabits forests in higher elevations, closer to the Andes.[19] Amazon species are epiphyte and the only Bifrenaria species with elongated rhyzome and ascendant growth. DescriptionBifrenaria generally are robust plants, of sympodial growth, which measure between ten and sixty centimeters height. Characteristics of an orchid to be classified under this genus are: to show roots of round section with thick vellamen; four angled fleshy pseudobulbs of one internode, often basally protected by dried steaths and with only one apical leaf, except for Bifrenaria steyermarkii, which occasionally has two;[20] plicate enervated leathery leaves, yet malleable and not exceedingly thick, with a pseudopetiole of basal round section; basal inflorescences bearing up to ten flowers, which seldom surpass the leaves length.[8] Bifrenaria flowers are fragrant or have a strong scent, they have sepals slightly larger than the petals, the lateral ones basally united to the column foot forming a calcar with truncated extremity; labellum variously shaped, often hairy, articulated to the column, with a longitudinal channeled callus often with a basal claw; the column is slightly arching, generally without wings or any other appendages, bearing a foot which the labellum is hinged to; they show two elongated stipes, hardy ever one, at least twice longer than wide, with salient viscidium, visible caudicles and retinacle in inverted positions; four hard superposed pollinia, protected by a deciduous incumbent anther.[10] The fruits are green, erect or pending, take about eight months to ripe and hold hundreds of thousand yellowish or brownish elongated seeds up to 0.35 mm long.[21] Among all the mentioned, the main characteristic to distinguish Bifrenaria form its related genera is the presence of the calcar on its flowers.[8] Other important qualities are the four sided unifoliated pseudobulbs besides the raceme inflorescence with to ten flowers.[22] Few is known about Bifrenaria species pollination. Apparently the only existing records report the presence of some large species' pollinaria observed on the back of male Eufriesea violacea, Euglossinae bees,[23] and of Bombus brasiliensis, Bombini bees.[24] Although there is no information of direct observation flower pollination, a research published in 2006 studied the micromorphology of the labellum of Bifrenaria species searching for substances useful to insects as food:[25] The absence of these substances on the densely pubescent surface of most Bifrenaria labelli in fact seems to indicate possible pollination by large bees as the mentioned before. Other indicator of this possibility is the strong smell emanated by some species, as B. tetragona, similar to the ones of other families flowers which are pollinated by these bees. The smaller species yet pubescent may be pollinated by smaller bees species ant the smooth ones which have strong colored flowers, as B. aureofulva, by hummingbirds.[25] Bifrenaria are comparatively easy to grow orchids. They should be preferably potted on well drained vegetable fiber because their roots and pseudobulbs get rotten easily when kept humid for long periods. As previously mentioned three different environments are needed in accordance to the species origin to successfully grow these plants. The large species need more light than the rest. The small species from Brazilian Southeast may be cultivated at the same medium temperature but under less 10 to 20% luminosity. Bifrenaria from Amazon forest need higher and more constant temperature and humidity than the other species. All species need more water and fertilizer during their active growth season.[8] Taxonomic notesThe genus Bifrenaria has been trditionallt classified under Bifrenariinae subtribe of tribe Maxillariae, Epidendroideae subfamily, however, the relationships among the several genera within this tribus are not well defined and changes are foreseen for next years. The genus closest to Bifrenaria is Rudolfiella. Other related genera are Teuscheria, Guanchezia, Hylaeorchis and Horvatia, besides the more distant Scuticaria and Xylobium. Recently is was suggested the unification of subtribes Lycastinae, Maxillariinae and Bifrenariinae, however, there is no consensus on the path to be followed.[26] Contrary to what was previously thought, the relationship among Bifrenaria al all these genera from Central America seems to indicate a primitive origin of Bifrenaria in Central America and its posterior dissemination towards the Southeast of Brazil, where it found fertile grounds to its more recent evolution.[10] In 2000, the first more complete molecular analysis on Bifrenaria species were made.[10] Sixteen species of this genus and six of close genera were studied searching for conffirmation of their phylogenetical position, besides the delimitation of each species and each of Bifrenaria's groups. The obtained results do not allow the acceptance of genus Adipe and, although they confirm Cydoniorchis monophyly, they dissuade their use otherwise six other genera should need to be created to fulfill the remaining fifteen species. They discuss also the convenience of splitting two species that are similar to each other and variable among themselves, with many hard intermediate forms hard to delimitate as B. charlesworthii and B. racemosa. It is also confirmed the position of B. steyermarkii out of Bifrenaria yet no genus name is suggested. The first species to be described, today classified as a Bifrenaria, was initially denominated Dendrobium harrisoniae by the author of its description, the English Botanist William Jackson Hooker,[27] now Bifrenaria harrisoniae. In 1827, Hooker described the first species of the small ones group, B. racemosa, however, classified it under the genus Maxillaria.[28] With this two publications started a long series of species descriptions and confusing genera about which many doubts existed during the two following centuries. The Royal Botanic Garden registers the submission of 69 species or ou varieties under genus Bifrenaria since the description of the first species.[4] Among these, twenty are generally accepted but only seventeen are well established, having no doubts about their true limits and classification. Thirteen species are accepted but now belong to other genera; and four or five, due to deficiencies on their descriptions, possibly will never be solved. The remaining species and varieties are synonyms of the accepted ones.[10] Few years later, in 1832, John Lindley proposed the genus Bifrenaria when describing its type species, Bifrenaria atropurpurea,[29] previously described by Loddiges as Maxillaria atropurpurea.[30] The name Bifrenaria comes from bi, two, and freno, brake, a reference to the shape of the two pairs of pollinia hold by separated caudicles presented by its flowers.[8] In 1837, Constantine Samuel Rafinesque, considering the noticeable vegetative difference between the few Bifrenaria known at the time, proposed the genus Adipe, based on B. racemosa morphology, described by Hooker few years before, to which added the description of a supposed new species, today considered its synomym, with the name of Adipe fulva.[31] On next year Lindley received a specimen of B. longicornis from Amazon, which was morphologically even more distant from the known species, however, he described it as Bifrenaria. Five years later, apparently not aware of Rafinesque's previous genus Adipe, changed his mind and suggested that this species should be classified under a new genus, Stenocoryne.[32] While six species were attributed to Lindley's genus Stenocoryne by several taxonomists during following years, the genus Rafinesque proposed remained forgotten until 1990.[4] Two species similar to Bifrenaria but that show a highly salient claw at the base of the labellum and lateral lobes abruptly divided, were then classified under this genus. In 1914, Rudolf Schlechter, based on Rudolfiella aurantiaca which presented the mentioned differences, suggested they should be classified under the genus Lindleyella, however this genus name was already occupied by plants of Rosaceae family.[33] Just thirthy years later, in 1944, the Botanist Frederico Carlos Hoehne, when working on the first revision of genus Bifrenaria corrected the suggestion of Schlechters. Hoehne initially proposed the genus Schlechterella to these species but, coincidentally, this name was also taken, now by Asclepiadaceae family plants.[34] Finally another name in homage to Schlechter was chosen, Rudolfiella, at the time with the number of species already increased to seven. On this revision, besides Rudolfiella, Hoehne divided Bifrenaria into two genera, therefore accepting the genus Stenocoryne proposed by Lindley but calling attention to the existence of Rafinesque's genus Adipe, which should have name priority over Lindleys and also raised doubts about the identity of several described species.[35] In 1990, Manfred Wolff resurrected the genus Adipe and to it submitted ten Bifrenaria species, besides the two already described by Rafinesque, however, not justifying his critters not revising the species.[36] Making the picture even more complex, in 1994, Karheinz Senghas, based on several characteristics shared only by B. tetragona and B. wittigii, described the genus Cydoniorchis to accommodate them.[37] In 1996, Gustavo Romero and Germán Carnevali transferred to Bifrenaria a species originally described by Schlechter as Maxillaria petiolaris, now classified as Hylaeorchis petiolaris.[38] On the same year, Vitorino Castro Neto published the most recent revision of Bifrenaria and its five sections, which is the classification used today.[39] SpeciesBifrenaria is formed by about twenty species[4] divided in two main groups of plants, large and small, with some visible morphological subdivisions highly confirmed by phylogeny. Large species: is the group originally classified as Bifrenaria. They present four sided pseudobulbs, with relatively short and erect inflorescence bearing up to ten fleshy large flowers but generally less. Usually the flowers are grouped and are fragrant or exhale strong scent. The labellum has three or four lobes and an elongated low callus. They are epiphytes, or often lithophytes. All originated in the southeast of Brazil. This group can be split in three subgroups:[10]
Small species: is formed by the plants that once belonged to Stenocoryne, or more accurately, Adipe, which normally are epiphytes. They present smaller and not as noticeably four sided pseudobulbs, and long and delicate inflorescence bearing a higher medium number of flowers than the large species, although also never surpassing ten. The flowers are smaller and not fleshy, with an entire labellum, or sometimes slightly lobed on the apex. These species take less luminosoty and more humidity than those and are not particularly fragrant. According to their morphology they can be split in four distinct subgroups:[10]
Other species: the remaining species are plants about which classification consensus has not been achieved: Bifrenaria maguirei, also classified under the genus Guanchezia, and Bifrenaria grandis, under Lacaena. Bifrenaria steyermarkii is a species highly different from all other Bifrenaria because its inflorescence is very long ant its flowers highly narrow, therefore it does not fit in any group, nevertheless the only other option of classification that has been published so far is under Xylobium what possibly is not a choice either. References
|
Daniel Mietchen 17:24, 4 June 2009 (UTC), David E. Volk 13:20, 7 June 2009 (UTC) |
6
| ||||||||||||||||||||||||||||||||||||
Miltonia is an orchid genus formed by nine epiphyte species and eight natural hybrids inhabitants of Brazilian Atlantic Forest, one species reaching the northeast of Argentina and east of Paraguay. This genus was established by John Lindley in 1837, when he described its type species, Miltonia spectabilis. Many species were attributed to Miltonia in the past, however, today, the species from Central America and from cooler areas on northwest of South America have been moved to other genera. Miltonia species have large and long lasting flowers, often in inflorescences with several of them. This fact, allied to being species that are easy to grow and to identify, make them a favorite of orchid collectors all over the world. Species of this genus are extensively used to produce artificial hybrids. Despite the fact that Miltonia is now a well established genus, most of its species were originally classified under other genera as Cyrtochilum, Oncidium, Odondoglossum, and Brassia. All were discovered between 1834 and 1850 with the exception of M. kayasimae, discovered only in 1976. DistributionMiltonia species range starts on the area of Missiones in the northeast of Argentina[1] and east of Paraguay[2] and spreads north along the Brazilian mountains of Serra do Mar and its branches up to the State of Pernambuco on Brazilian northeast. They occupy mostly areas between 200 and 1,500 meters of altitude meters, however the majority of the species are more often found about 600 to 900 meters. Miltonia species can be found from shady areas inside the forest to areas more exposed to the sun, however never are under full sunlight; usually in ventilated places where they receive plenty humidity during the night and early morning. They are always epiphyte and, because they grow very fast, each pseudobulb originating two new growths every year, they soon form large colonies.[3] Miltonia russelliana and M. flavescens are the ones with the widest dispersion and found at lower altitudes. M. flavescens is the only species that exists in countries other than Brazil and is also the one that spreads farther north. M russelliana range starts on Rio Grande do Sul and ends at Bahia State. M. regnellii is also widespread although does not go northern than Rio de Janeiro. M. moreliana is a species more common at lower altitudes and warmer areas existing from Rio to Pernambuco. Miltonia candida, M. clowesii and M. spectabilis are restricted to the four states of Region Southeast of Brazil. Miltonia cuneata is just from São Paulo and Rio and the one that grow at highest altitudes. M. kayasimae is the only species really rare; it has been found just a couple of times in a very restricted area close to Salesópolis, in São Paulo State. The mountains area between São Paulo and Rio de Janeiro, where almost all species do exist may be considered the center of distribution of Miltonia.[4] DescriptionMiltonia are comparatively medium large orchid plants reaching about fifty centimeters height. They present subcaespitous growth, that means their pseudobulbs are not tightly packed but slightly spaced by a rhyzome, that is longer than on caespitous plants, with length between two and five centimeters. Their roots grow along the rhyzome in high numbers. They are white, comparatively thin, usually short and hardly branched. The rhyzome is covered by dried imbricating steaths which get increasingly larger at the base of pseudobulb becoming articulated foliar steaths that partially cover them. The pseudobulbs and leaves vary in color from yellowish bright light green to olive green depending on the species and to the amount of sunlight they are exposed to. They may be more oval and laterally highly flattened to slightly tetragonal and elongated and almost always bear two apical leaves. The leaves are narrow, flexible and hardly larger than three centimeters wide and forty long with the apexes rounded sometimes slightly pointed. Some species are about half of this size. The inflorescences are one or two per pseudobulb, shoot from their bases behind the protecting steaths. They are erect and never branched, often longer than the leaves, bearing from one to twelve moderately spaced flowers that open at the same time or in succession holding three or four opened all the time, when the older fades a new one opens. The older flowers of species with white lips that open in succession usually get yellower about the time the next flower opens although they still last one more week before fading. The first to bloom is M. cuneata, during late winter, but the majority of species bloom from late spring to late summer.[5] The flowers of Miltonia vary from four to fifteen centimeters across; the larger are the ones with fewer flowers. Their colors vary from entirely white and pink to dark purple, pale yellow or lilac when plain, or they may highly spotted but then usually they are greenish or brownish with a contrasting labellum often white with purple dots, stains or veins close to the base.[3] The petals and sepals shapes are highly variable from species to species but always somewhat similar to each other within a species. They may be erect and flat or sometimes less open. The labellum is simple or very slightly lobed, usually very wide and showy without salient calli although normally showing more or less subtle keeled thickenings close to the base, usually of different colors; it is much larger and wider than the other segments, often flat but in M. candida embraces the column and in all species it is slightly fused to the column at their bases. The short column does not have a foot and presents two lateral auricles sometimes merged to each other through a fringe that surrounds the superior edge of the clinandrium. The anther is apical and bears two yellow hard pollinia. They possibly are pollinated by bees.[6] Taxonomic notesThe first species to be described, among the ones today classified under the genus Miltonia, was originally published by John Lindley, in 1834, as Cyrtochilum flavescens. In this description Lindley notices that the flowers of this species turn orange color when drying and, for some confusion regarding the origin of the species, attributes it to Mexico instead of Brazil.[7] Two years later Lindley described another Miltonia species but, then, under the genus Oncidium, as O. russellianum in homage to Duke of Bedford. When describing this plant, Lindley considered it as a transition species pointing out that it was very different from the average Oncidium because of its purple colors and undivided lip.[8] In 1837, Lindley received from Mr. Loddiges and from George Baker two other specimens of a very distinctive new species. Recognizing then this should in fact be a new genus, he proposed the name Miltonia to it as a homage to Lord Fitzwilliam Milton, an English orchid enthusiast. Lindley states then that the limits between a number of Oncidiinae genera, Cyrtochilum, Oncidium, Odondoglossum, Brassia and Miltonia, at that time classified as Vandaea, were yet to be perfectly established; although closely related, the differences should possibly be: Oncidium has a column with two ears and labellum distinctively lobed; Miltonia has a column with two ears and an entire labellum partially united to the column base; Odontoglossum and Cyrtochilum have winged columns and entire labelli but the former has it partially united to the column; and Brassia does not have any appendages on the column. It is interesting to notice that despite Lindley described the genus Aspasia in 1833, which is the most closely related to Miltonia, both by flower and vegetative morphologies, he did not mention it on Miltonia description.[9] Three other Botanists were working with Miltonia species around the time Lindley described this genus. All recognized these plants should be classified under a new genus and, as communications were slower then, all proposed new genera: Knowles and Westcott also received also a plant of M. spectabilis and, just one month after Lindley, proposed for it the genus Macrochilus, calling the species Macrochilus fryanus;[10] the other one was Rafinescque who, in 1838, decided that the Oncidium russellianum already described by Lindley in 1836 should be under another genus and created for it the genus Gynizodon.[11] Both Macrochilus and Gynizodon are synonyms of Miltonia and no other species has ever been submitted to them.[2] As Miltonia species are common plants, comparatively large, with also large flowers of bright colors, that, moreover, are spread mostly over an area of early settlements in Brazil all species but one were already described in 1850; six of them by Lindley, M. regnellii by Reichenbach[12] and M. moreliana by Achille Richard.[13] Despite the early description of M. moreliana in 1848, and two other as M. rosea by Lemaire in 1867,[14] and as M. warneri by George Nicholson in 1886,[15] Arthur Henfrey reduced it to a variety of Miltonia spectabilis in 1851,[16] and as such it was considered until 2002, when Cássio van den Berg reestablished it as a distinct species.[17] The last Miltonia species to be discovered was M. kayasimae, found by an orchid collector not far from the city of São Paulo, in an area around nine hundred meters of altitude nearby the top of Serra do Mar mountains. It was named after their collector by Guido Pabst in 1976. So far very few plants were found, all living at the same area.[18] Since the genus Miltonia was established, many species, now classified under a number other genera, were submitted to it. The most noticeable cases were four of the five species of Miltoniopsis,[19] a genus proposed in 1889 but only really accepted in 1976.[20] Despite its somewhat similar flowers, Miltoniopsis are from cooler forests on the Andean slopes closely related to Cyrtochilum and only remotely related to Miltonia. Also five of the six Miltonioides species were occasionally considered as Miltonia until 1983 when Brieger and Lückel proposed this genus for them.[21] These are species of more delicate and narrower flowers, from Mexico and Central America, which some taxonomists claim might be better classified under the genus Oncidium to whom they are closely related.[22] The last common species which was occasionally classified under Miltonia is Chamaeleorchis warszewiczii,[23] which is related to Oncidium and some taxonomists identify as Oncidium fuscatum. In 1983, Brieger and Lueckel, considering that four species of Miltonia, M. candida, M. cuneata, M. kayasimae and M. russelliana, show the junction of the labellum with the column in a different angle than the other species, proposed the genus Anneliesia for them.[24] Although this four species form a small sister clade to the rest of Miltonia species, the difference did not seem important enough to justify the acceptance of this new genus, therefore this proposal has not been generally accepted by the scientific community.Cite error: Closing Molecular analysis show that Miltonia most closely related genus is Phymatochilum and then Aspasia, Brassia and Ada, which are the most important genera included in this that is one of the eight clades that form the subtribus Oncidiinae of tribus Cymbidieae.[22] SpeciesThe species of Miltonia show many differences to each other and are very easy to identify, therefore, just the most evident differences are mentioned here; more details are given on individual species articles. The species are presented here according to their morphology and this order keeps no correspondence with phylogenetic relationships. Regarding vegetative morphology Miltonia moreliana and Miltonia spectabilis can be immediately separated from the rest because their much flatter pseudobulbs, longer rhyzome and inflorescences completely covered by flattened bracts that bear only one highly flat flower. These are the species with largest flowers in the genus. They are closely related and usually are recognized because the flowers of M. moreliana usually have dark purple petals and sepals and the lip of a lighter bright purple while M. spectabilis has very light purple or white petals and sepals and a purple veined labellum, however, the real technical difference among the species is on the proportions of their segments which are much wider. Despite colors are often mentioned to identify species they are not accepted by taxonomy as enough to establish distinct species by themselves.[17] All other Miltonia species have similar vegetative appearance and only can be positively identified by their flowers. Three species are very unique: Miltonia flavescens has the most narrow flowers, almost star shaped, with all segments of straw color with some purple bots on the base of petals and sepals which are more intense on the labellum almost forming stripes;[1] Miltonia candida is the only species with a labellum that embraces the column in a way that reminds the Cattleya species;[26] Miltonia russelliana is the less showy of Miltonia species because its sepals and petals do no really open, being always bent over the column, revealing only the lighter tip of its purple labellum.[8] Miltonia regnellii shows the widest flower color variation among all Miltonia species; they can vary from white to yellow, pink and lilac with labelli also varying from white to dark purple. The flowers open in succession and slightly resemble the ones of M. spectabilis although much smaller. They actually are the Miltonia species with the smallest flowers.[12] Miltonia kayasimae and Miltonia cuneata are somewhat similar and possibly are closely related, both have straw color petals and sepals almost entirely covered by large brown stains and white labelli, however, they show different proportions on the flowers segments. M. kayasimae has much wider petals and sepals and smaller labellum which, moreover, has a larger and more salient and complex entirely purple callus on its base which is delicate, more straight and simple, and just occasionally purple dotted on its apex on M. cuneata.[18] Miltonia clowesii has the same color pattern of M. russelliana with light yellow greenish brown sepals and petals completely covered with large darker dots or stains and labellum of bright purple at the base and lighter apex, however here they are whiter. On the other hand, M. clowesii flowers pointed segments are larger and wide opened making it to remind a spider.[27] Natural hybridsConsidering its limited number of species, it is surprising that eight natural hybrids of Miltonia are currently known, a number that almost equals the number of species and also implies that the most important pollinator of the majority of the species possibly is the same. As the crossing of two species uses to produce variable plants most of these hybrids have been described more than once and some have three or four synonyms. M. spectabilis is the species which has produced the largest number of hybrids, five: Miltonia × bluntii when crossed with M. clowesii, Miltonia × cogniauxiae with M. regnellii, Miltonia × flava with M. flavescens, Miltonia × leucoglossa with M. candida and Miltonia × rosina with M. cuneata,[2] furthermore it is possible there is also one with M. moreliana, which has not yet been described because M. moreliana itself was earlier considered a variety of M. spectabilis. M. candida, besides the hybrid already mentioned with M. spectabilis, also produced two others: Miltonia × binotii with M. regnellii and Miltonia × lamarckeana with M. clowesii.[2] The remaining hybrid, Miltonia × peetersiana used to be considered a synonym of M. × bluntii but because M. moreliana is now a species distinct from M. spectabilis it is its hybrid with M. clowesii, which has entirely purple flowers instead the one with light brown petals and sepals.[28] CultureDespite being easy to grow Miltonia species tent to be subject to spots on their thin leaves generally caused by fungi proliferation and normally, when exposed to the amount of light they need to a full bloom, their foliage gets a little bit too yellow colored, although they should never be exposed to full sunlight. Finding the right balance of light exposure to avoid the yellow leaves but still produce nice blooming is important and with some tentatives the grower will succeed. They are not much sensitive to temperature but it varies according to their origin M. cuneata being the one that grows cooler and M. moreliana the warmer grower all under intermediate temperature with at least 10ºC of variation between day and night. Despite they show a rest period after blooming, Miltonia always need to be watered, more abundantly during active growth. they need at least 65% of humidity and good ventilation all the time. Moderate weekly fertilizing with a balanced formula is beneficial during active growth. They may be potted in a compost of half-chopped Sphagnum, peat, and some medium sized lumps of charcoal, or mounted on plaques of vegetable fiber, however if mounted they will need more frequent waterings.[29] References
|
Daniel Mietchen 17:24, 4 June 2009 (UTC) | 3
| ||||||||||||||||||||||||||||||||||||
Scuticaria is a genus in the orchid family formed by nine species of showy flowers and long cylindrical leaves. They are epiphytic, occasionally lithophytic or terrestrial, that grow pending and are cespitosus, or reptant and ascending, which exist in three isolated areas of South America, the Amazon Forest in Ecuador and the Serra do Mar and Serra da Mantiqueira mountains in Brazil, both in shady and sunny places. The genus Scuticaria has been traditionally placed close to Maxillaria but recent research shows they are more closely related to the genus Bifrenaria. Despite their interesting appearance, the are hardly seen in nature and, because they culture is complicated, they are not common in private collections and orchid shows either. No other use for these species is reported besides ornamentation. Because it is a well established genus, formed by few species that are reasonably easy to separate, there were few publications about them during the last decades. Distribution and habitDespite there are few species, Scuticaria inhabit varied climates, disperse in a very uneven way though all countries of South America northern to Bolivia, this excluded, and also in areas of Mata Atlântica in Brazilian Southeast. No species is common in nature, being just occasionally or even rarely found. The species with wider range is Scuticaria steelei which inhabits open clearings at higher elevations of central Amazon, jungles known as matas de terra firme, up to eight hundred meters of altitude.[1] Although this species occupies wide area, it is not found very often.[2] Another species from Amazon, however, in a much more restricted area, just in Guyana, in places where the altitude is lower and the humidity is higher, is Scuticaria hadwenii var. dogsonii.[3] Endemic in another area of Amazon, separated but not that far from the habitat of Scuticaria steelei, on southeastern Ecuador, close to the place where the Andes starts, in humid and slightly colder forests, on the mountains up to 1,300 meters of altitude, it is found Scuticaria salesiana.[4] Under the same conditions but in wider areas, that encompass the southeast of Ecuador and northeast of Peru, lives S. peruviana.[5] All species from Amazon are always epiphytic. The remaining species inhabit the area occupied by Brazilian Atlantic Forest. The only species that can be found widespread through several states is Scuticaria hadwenii, in the humid jungles of Serra do Mar from Santa Catarina to Bahia States,[6] generally found living epiphytic at middle height over thick tree stems.[7] Other species occasionally found, although often under living litophytic over rocks and gatherings of fallen leaves in sunny areas of the mountains of São Paulo and Rio de Janeiro, is S. strictifolia.[8] Scuticaria irwiniana, second and last rupicolous species, exists only on the mountains of Minas Gerais State, found in sunny or shadier places up to two thousand meters of altitude.[9] Two are the species from Espírito Santo State, S. novaesii and S. kautskyi, both endemic of restricted areas in the dry jungles of the countryside.[10] The last Scuticaria species is S. itirapinensis, which has been found only a couple of times in the west-central dry woods of São Paulo State, in an area which has been highly deforested, close to Itirapina. There are no records or reports on this species, both in nature and under culture, during the last twenty five years. It is speculated about the possibility of its extinction.[11] DescriptionThe species subordinated to genus Scuticaria are characterized by being plants of thick cylindrical roots covered by thick vellamen. Their stem is formed by a ordinarily short rhizome, slightly elongated in some species; and by cylindrical almost inconspicuous pseudobulbs of the same diameter or slightly thicker than the unique leaf born on their apexes, because they generally are covered by small dried scaling steaths. The leaves may be erect or pending up to one meter long. The inflorescences grow from the said steaths and almost always bear just one flower, exceptionally two in one species, and always is much longer than the pseudobulbs, bearing showy yellow, orange, purple or greenish flowers, with petals and sepals plain, stained or striped, usually by light brown but also by diverse combinations and shades of the other mentioned colors. Ordinarily the labellum presents contrasting colors, frequently with white areas.[8] The flowers are large, wide open, and last during about two weeks.[2] They have sepals of similar sizes and form an almost invisible chin with the column foot. The petals may be similar to the sepals but smaller, or much smaller and with a much narrower base, occasionally showing different patterns or colors. The labellum articulates with the column, is trilobed, with comparatively small lateral lobes and larger terminal, which has variable shapes with diverse patterns and a callus under to column. The later is é semi-cylindrical, slightly arching, erect and thick, without any kind of appendix, ending in an apical anther and elongated in a small foot at the base. The flowers bear to pairs of pollinia of different sizes. The caudicle is narrow and the retinacle is small. The fruits resemble the ones of Maxillaria.[8] There are no observation records of pollinators activities but Scuticaria are supposedly pollinated by Euglossini bees.[7] Taxonomic notesIn May of 1837, the English Botanist William Jackson Hooker received a drawing and a dried sample of a plant, sent by an orchid grower from Liverpool, together with a note explaining that the plant arrived from Demerara, in Guyana, in July of the preceding year. Hooker described this species, classifying it under the genus Maxillaria, calling it M. steelei, in homage to its discover. In his description, Hooker affirms that the plant is highly interesting and an excellent addition to the known epiphytic species because it shows cylindrical leaves almost one meter long, different from anything ever found.[12] few months later, John Lindley published again the species Hooker described, however, adding more information. Two years earlier, several plants had been sent from Demerara and Lindey reports that he had previously informally classified this species as Maxillaria flabellifera which, under this name, could be found in several orchid collection in England. Because he had not yet described this species, he accepts the priority of the name chosen by Hooker. Lindley adds, however, that he had some doubts about the classification, of a species so different from any other known so far, under the genus Maxillaria.[13] In 1843, Lindley published a revision of a group of orchids classified as tribus Maxillaridae, then subordinated to Vandeae, a subfamily of Orchidaceae at the time. In this revision, he indicates that much work is needed till the limits between each genus within this tribus can be established and states his doubts regarding some of the new genera he was proposing, despite being very sure of other ones. Lindley suggested the division of Maxillaridae in twenty five genera, being Scuticaria one of the genera he considered well established. When describing this new genus, Lindley based on morphologic characteristics of Maxillaria steelei Hook., selected as the Type species of Scuticaria with the name Scuticaria steelei.[14] This name comes from Latin scutica, flagellum, in reference to the long cylindrical leaves that the species of this genus show, similar to the leather whips used to punish.[8] Strangely, because he published the genus Scuticaria many years earlier, in 1851, Lindley described another species now considered part of this genus, classifying it under Bifrenaria. It is speculated that possibly because it was found in Brazil on the same area in the southeast where most of Bifrenaria were common, or because he believed that two species separated by so long distance belonged to the same genus. It was Scuticaria hadwenii.[15] Few months later, Jules Émile Planchon corrected Lindley moving it to the genus where it is subordinated today.[16] In 1851, the only two common Scuticaria species were described and the genus well established, therefore no later confusion about the classification of any species subordinated to this genus ever happened.[17] Almost one century passed before any important new information were published. In 1881 Heinrich Gustav Reichenbach described Scuticaria dogsonii, originated from Guyana,[3] but in 1892, Berthold Stein, considering that the only difference it shows from Scuticaria hadwenii is the fact it bears two flowers each inflorescence, reduced it to a variety of the later.[18] In 1903, Célestin Alfred Cogniaux, when revising all orchids species from Brazil, cites two other varieties of Scuticaria hadwenii which, because just show color differences, can not be accepted as such today.[19] Finally, in 1947, Frederico Carlos Hoehne described a new species, Scuticaria strictifolia, yet similar to Scuticaria hadwenii, although showing some slight differences on the labellum structure, besides their normally lithophytic habit and erect leaves.[20] If few species were known so far, after 1968 the number of described species triplicated. All species described during the later years are uncommon and inhabit restricted areas, some are very rare or even supposedly exctinct. In 1968 Robert Louis Dressler described Scuticaria salesiana, discovered in Ecuador in an area far apart from the other Scuticaria range.[4] In 1972, Guido Pabst described Scuticaria kautskyi, found in Espírito Santo State, [21] in southeast Brazil and, during the following year, published two species at once, S. itirapinensis and S. irwiniana.[22] In 1982, other species was discovered in Espírito Santo, Scuticaria novaesii.[10] The last described species was S. peruviana, found in Peru in 2002, in the same region of S. salesiana, to which it is related.[5] Despite Lindley indicated the possibility of Scuticaria being closely related to Bifrenaria when he initially described S. hadwenii under this genus, all later taxonomists always included Scuticaria on the same group Maxillaria were.[23] It was just in 2000 that the first proofs of Scuticaria closer proximity to Bifrenaria started being published.[24] In 2002, a detailed research about the phylogeny of Bifrenaria performed molecular analyses on two Scuticaria species while chosing them as out groups. This study claims that the phylogenethic internal relationships among Scuticaria species so far remain unknown.[25] It is known that other orchid genera bearing cylindrical leaves devolved this sort of leaves as a defense to climate changes their habitats were going through along the eras. Terete leaves are capable of much more water and nutrients and to face longer drought periods than species bearing thin leaves, on the other hand, almost all epiphytic species presenting the former type of leaves show more or less atrophied pseudobulbs since the leaves carry on its accumulating role. It is a supposition that Scuticaria species should have once inhabited much drier through their evolution. Because most of the species are found in shadier and more humid species now, this may one of the reasons why their culture uses to be complicated, possibly because the delicate balance they reached in nature is broken. For the same reason it is supposed their frequency in nature is only occasion or rare.[7] SpeciesBecause of its highly particular morphologic characteristics which allow immediate identification, their restricted species distribution, and their comparatively low variability, since the genus Scuticaria was established by Lindley, only then species were formally described and it has never been great confusion separating each species. Form these ten, nine are generally accepted, the tenth being ordinarily considered a variety, and, under this condition, also accepted.[17] For identification purposes, the species can be split as follow: Only two species present erect leaves and are the only ones frequently found as lithophytes, Scuticaria irwiniana, easily known recognized because of its flowers without any stains on the internally entirely purple and externally whitish sepals and petals, with white labellum, striped of purple. This species generally can be identified even without flowers because of its reptant, slightly ascendant growth, and longer rhyzome than any other species.[22] Scuticaria strictifolia also has erect leaves but occasionally, when cultivated under insufficient light, their leaves can be narrower and slightly bent making the distracted observer find hard to differentiate it from S. hadwenii.[8] The Brazilian taxonomist Guido Pabst considered this species a variety of the later.[9] All species remaining are ephiphytic with pendent habit. Scuticaria hadwenii, due to its several more or less isolated groups of populations along Serra do Mar, mostly on the west side of this chain of mountains, spreading throughout the interior highland in some states of Brazil, is the Scuticaria species that presents most variable colors.[8] It can be separated from S. strictifolia because shows leaves always pending, flowers of more vivid colors and by the interior of the labellum, which ordinarily is more pubescent. There is a variety denominated dogsonii, native from Guyana, which is more floriferous.[3] The two other species from Espírito Santo State are highly different to each other. Scuticaria kautskyi usually has more or less uniform orange color on its sepals and petals, with their bases slightly lighter and dotted of greenish yellow. Their labellum is white showing few colored drawings and narrow terminal lobe, slightly deflected.[21] The other species from this state, Scuticaria novaesii presents flowers with green-yellow segments, intensely spotted with dark brown and wide and flat labellum terminal lobe, with clearly marked by radial multicolored lines.[10] Scuticaria itirapinensis, the last species of brazilian southeast, is the one that closely resembles Amazonian Scuticaria steelei, although it can be easily separeted because of its strong yellow flowers and much shorter leaves, besides slight differences on the proportions of floral structures.[22] A Scuticaria steelei presents entirely pale yellow flowers, completely covered by spaced small darker stains, However in is not even necessary to observe the flowers to identify it as their leaves are about one meter long, and there are references of plants measuring almost one and a half meter.[2] The last two Scuticariaare isolated in forests of Peru and Ecuador and are similar to each other. They are different from all other because of the proportions of floral segments. The labellum is much larger when compared to their sepals and petals than it is on other species. Moreover, their petals are striped of brown and much smaller than the sepals, showing a greater difference than it is found on the other species. From each other, they can be separated mostly by the shape of the labellum. Scuticaria salesiana presents more rounded intermediate lobe, and Scuticaria peruviana has it more rectangular, with the apex truncated, almost in a straight line.[5] In 2008, a new species of Scuticaria, S. bahiensis has been described from Bahia state in Brazil but so far it remains mostly unknown.[26] CultureIn his book Flora Brasilica, the Brazilian Botanist Frederico Hoehne strongly recommended the culture of Scuticaria species because of their beautiful flowers and interesting vegetation, however, soon later he admits that all species then cultivated by São Paulo Botanic Garden had died after two or three years. Indeed, he claims that to successfully grow them a special environment needs to be created.[8] These plants are not easy to maintain under culture. Only recently, with the help of modern technology, timers and foggers that keep the humidity constant, the growers have been finally capable of keeping them out of their natural environment for several years. There are four different sorts of culture according to the origin of each species. S. steelei and S. hadwenii var. dogsonii are the species that need higher temperature and humidity. The two rupicolous species, S. irwiniana and S. strictifolia ate the ones which need more light and constant ventilation besides drier culture conditions.[22] S. peruviana and S. salesiana take slightly cooler temperatures than the other species although still need humidity mostly during the early morning hours.[5] The other species need less light than the mentioned ones. All species should be preferably mounted on plaques of vegetable fibers because of their pending habit, the rupicolous species may alternatively potted in well drained pots. Scuticaria are delicate plants that like to remain untouched during several years because their roots easily resent on replants.[7] References
|
Daniel Mietchen 17:24, 4 June 2009 (UTC) | 3
| ||||||||||||||||||||||||||||||||||||
| Torture: Add brief definition or description Torture (Read more...) |
Howard C. Berkowitz 14:32, 5 June 2009 (UTC) | 3
| ||||||||||||||||||||||||||||||||||||
Milpa agriculture is a form of swidden agriculture that is practiced in Mesoamerica. Traditionally, a "milpa" plot (from the Nahuatl word for "corn field") is planted with maize, beans, and squash (known as the Three Sisters) and might include a variety of other plants. These plots are planted for two or three years and then allowed to lie fallow for some years in order to restore the fertility of the soil. Milpa agriculture varies somewhat by region and it has changed in a variety of ways in different areas but it remains an important part of life for millions of people throughout Mesoamerica. Maize and beansMaize and beans, which are the staples of the Mesoamerican diet, complement each other in terms of the health of the fields as well as the health of the people who eat them. Maize requires high levels of nitrogen in the soil to grow properly and quickly depletes the soil if planted alone. Bean plants (genus Phaseolus), on the other hand, are high in nitrogen and their presence extends the life of the maize plot significantly by helping to keep nitrogen levels healthy. One might say that the maize repays the debt it owes to the beans by providing stalks for the bean plants to cling to as they grow. Squashes, generally grown between the rows of maize stalks, also figure into this symbiotic relationship, as they cover the ground in between the rows of corn and help to keep weeds down. Maize and beans also compliment each other nutritionally. If eaten alone, one would need to consume large amounts to fulfill human dietary requirements but when eaten together, they achieve "protein complimentarity."[1] That is to say, a person needs less total food if the two are eaten together. This diet is further supplemented by several varieties of squash and other foods that are planted along side the maize and beans. In many areas, the "Mesoamerican trio" (maize, beans and squash) is complemented by wild or semi-domesticated plants that grow in and around the milpa. Planting and harvestingMilpas are traditionally cultivated using the swidden, or "slash and burn" system. The forest is cut, allowed to dry and then burned. The left over ashes are then mixed into the soil as a fertilizer. Maize and beans are planted together in the same hole while squash is planted separately, in between the rows of maize. Other cultigens may be planted in a separate section of the field or scattered among the maize. The fields must be weeded several times throughout out the season. This is backbreaking work, especially in areas where a milpa is likely to be planted on the slope of a mountain several hours walk from home and it is usually done mostly by hand with the help of machetes and hoes. Later in the season, weeding is less needed, as the broad leaves of the squash plants keep unwanted plants to a minimum and the maize grows out of reach of the weeds. Beans and squash are harvested as they ripen. As the maize ripens, a few elotes (or fresh cobs) may be picked to be eaten right away but most of the plants are bent near the top and the cobs are allowed to dry in the field. Once they are dry, they are collected and stored for later use in traditional Mesoamerican foods like tortillas and tamales. The dry stalks are often collected and sold at market as fodder for animals and the husks are frequently saved for use in preparing tamales. The weeds and left over corn stalks from the previous season will be burned to prepare the fields for planting for one or two more years but then they will be left to fallow for as much as ten or more years. This practice has been abandoned in many areas because of the shortage of land that is available to many families. Instead, animal dung or ashes from the hearth at home may be added to those mixed into the soil and many farmers have turned to industrial fertilizers that allow them to cultivate the same plot year after year. The ritual life of the milpaSeveral important rituals are performed at key points during the growing season. The timing of these rituals varies by region and altitude due to variations in climate and the length of the growing season but they are invariably tied to significant events in the ritual calendar. Among the Maya, agricultural rituals are performed at the full moon[2] while in central Mexico, each ceremony is associated with one of the eighteen months in the solar calendar and occur at periods of twenty days.[3] Ceremonies are performed at each major stage in the development of the maize, beginning with the planting and concluding with the harvest. Agricultural rituals also vary in form according to local traditions, but common themes are found throughout Mesoamerica. One such theme is the ritual reenactment of the creation of the world by marking the four cardinal directions. Among the K'iche' of highland Guatemala, offerings are made at the four corners of the milpa. The Kekchi plant maize in the center of the field first and then plant in each of the fours directions.[2] In Tepotzlán, the Nahuas place crosses made from pericón at the four corners of the field.[3] And in the Sierra Madre of northern Mexico, the Tarahumara open their work parties by scattering tesguino, or corn beer, to the four directions.[4][5] Other themes include sacrifices and offerings to indigenous deities or Catholic saints and prayers for rain. Maize also figures prominently in ritual activities that are not directly connected to the cultivation of the milpa. In Amatlán, Nahua villagers say, "Corn is our blood,"[6] meaning, "corn defines our way of life" in everything from ethnic identity to every day activities. In fact, the Popul Vuh reveals that the first mothers and fathers of the Maya were actually formed from maize.[7] Maize, and especially the intimate connection between maize and humans, arises again and again in both ancient and modern myths. Modern trendsOne consequence of the changes in land ownership patterns introduced through Spanish colonial society and some of the agrarian reforms carried out by the modern states in the region is that many families do not own enough land to produce all of the food that they need in a year. Consequently, the practice of fallowing the fields has disappeared or nearly disappeared in many areas. Today, many farmers use chemical fertilizers to increase their fields’ production and engage in other economic activities to earn enough money to provide for their families. Even when farmers have access to enough land to feed their own families, they are often unable to market their surplus. Trade liberalization and market deregulation policies adopted throughout the region in the 1980s and 1990s have meant that maize and other staple crops produced on a very large scale by U.S. corporations are frequently available at lower prices in local markets than the products of local farmers.[8] Thus, many farmers have also converted their fields to other crops. Today, cash crops destined for export to the U.S. include broccoli, snow peas, cucumbers, Brussels sprouts, and carnations. Despite the market pressures felt by small scale farmers in Mesoamerica to turn away from traditional agriculture, milpas may hold information that can help improve modern agricultural methods. Monocrop agriculture creates artificial growing conditions that are significantly less biologically diverse than the natural ecosystems that they replace. This results in rapid depletion of the soil, which is generally counteracted by the application of chemical fertilizers that return nutrients to the soil but may have damaging effects in the long-term. Though it is unlikely that the balancing diversity of milpa agriculture can be reproduced on an industrial scale, the indigenous knowledge embedded in its design may provide some guidelines for improving industrial techniques. "By studying [the milpa's] essential features," writes Charles C. Mann, "researchers may be able to smooth the rough ecological edges of conventional agriculture."[9] Notes
|
Milton Beychok 00:15, 29 May 2009 (UTC) | 1
|
Current Winner
To change, click edit and follow the instructions, or see documentation at {{Featured Article}}.
| The metadata subpage is missing. You can start it via filling in this form or by following the instructions that come up after clicking on the [show] link to the right. | |||
|---|---|---|---|
|
| Halobacterium sp. NRC-1 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
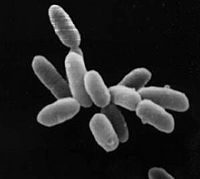 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Halobacterium sp. NRC-1 |
Halobacterium sp. NRC-1 is a non-pathogenic, halophilic archaea that thrives all over the world in high salt environments, including salt production facilities, brine inclusions in salt crystals, natural lakes and ponds, and salt marshes. Prior to 1990 H. NRC-1 was classified as an archeabacterium under the prokaryote kingdom in the two-empire system which consisted of eukaryotes and prokaryotes. Since 1990 the prokaryotes were split into bacteria and archaea due to their different evolutionary paths and biochemical differences.[1] Like all archaea H. NRC-1 has no nucleus or organelles within the cell, and like other archaea, have evolved many metabolic pathways to allow it to survive in extreme environments.[2]
Halobacterium sp. NRC-1 is motile using both flagella and gas vesicles, and respond to their environment by moving toward or away from chemicals in a process called chemotaxis. Additionally, they can move toward or away from light in a process called phototaxis by utilizing their sensory rhodopsins. Phototaxis is an advantageous ability for halobacteria because it enables them to swim away from the high levels of ultra violet and ionizing radiation that they are exposed to on a daily basis. Halobacterium sp. NRC-1 reproduce by binary fission and grow best in a 42 degree Celsius, aerobic, high salt environment.
Halobacterium sp. NRC-1 is very easy to culture in the lab and its genome has been completely mapped and sequenced. Whole-genome DNA microarrays are available to investigate gene expression. This makes it an excellent model microorganism for research in basic cellular processes, gene expression and as well as for teaching.
Genome structure
The genome of Halobacterium sp. NRC-1 was published in 2000. Since that time scientists have done extensive research to gain insights into both its extremophilic abilities as well as its biological processes. In order to survive in its environment, H. NRC-1 has developed amazing capabilities to repair its own genome, one being a precise base excision repair system. Homologous recombination plays an important role in DNA repair as well. Its ability to repair its chromosomes after extensive damage is only exceeded by the extremely radiation resistant Deinococcus radioduransbacterium.[3] In fact, H. NRC-1 was found to display a significant number of unique homologs with this bacterium, which suggests that they might have been acquired through lateral gene transfer.[4] This microorganism also displays other types of defense mechanisms. In combination with its saline environment which provides some protection from UV radiation, the proteins that are produced by H. NRC-1 were found to be highly acidic. This low pH helps the proteins resist the denaturing effects of the high concentration of salts that surrounds it.[5] [6]
Halobacterium sp. NRC-1 contains the smallest genome to date among the halophiles.[6] It is 2,571,010 bp in size, and is composed of a large GC-rich chromosome (2,014,239 bp, 68 % G+C), and two smaller extrachromosomal replicons, pNRC100 (191,346 bp) and pNRC200 (365,425 bp), with 58–59 % G+C composition. The large chromosome contains 2,111 genes, pNRC200 contains 374, and pNRC100 contains 197. Of the 2,682 genes in the genome, only 1,658 coded for proteins that had significant matches to the genome database. A significantly smaller fraction of the genes on pNRC200 and pNRC100 matched to genes of known function in the databases. The genes on the large chromosome had a 45% match rate, while pNRC200 had a 32% match rate and pNRC100 had only 26% of its genome match up to genes of known function. 52 RNA genes have also been identified. Interestingly, about 40 genes that are located on the small replicons, pNRC100 and pNRC200, code for functions likely to be essential or important for cell livelihood (e.g. thioredoxin and thioredoxin reductase, a cytochrome oxidase, a DNA polymerase, multiple TATA-binding proteins (TBP) and transcription factor B (TFB) transcription factors, and the only arginyl-tRNA synthetase in the genome. In fact, these two replicons share 145,428 base pairs of identical DNA.[4]
After the publication of Halobacterium sp. NRC-1’s complete genome, the mapping of another closely related microorganism, Halobacterium H. Salinarum, was published as well. Interestingly, genomic comparisons between these two halophiles showed that the large chromosomes were virtually identical to one another while the smaller replicons carried different genes.[7] This information has cast new light on how the species differ from each other and the role of replicons in these organisms. While much of the published literature on Halobacterium sp. NRC-1 refers to the smaller genetic elements as replicons or megaplasmids, their characteristics don't really fit the definition of these terms. Replicons are considered to be exact copies of specific sequences of an original DNA or RNA genome, or even a whole copy of the original genome. Plasmids are referred to as being small extra chromosomal DNA elements that carry relatively few genes that code for genetic information that is not essential to an organism's biological processes. Considering that the genetic information carried on these replicons not only code for information that is essential to the organism's survival, but also contain nucleotide sequences that are not identical to that of its larger chromosome, scientists are beginning to refer to them as "minichromosomes" rather than megaplasmids or replicons.[6]
Cell structure and metabolism
Halobacterium sp.NRC-1 is a rod shaped aerobic chemoorganotroph which uses organic molecules as its source of energy, carbon and electrons. This halophile possesses facultative anaerobic and phototrophic capabilities as well. Research has shown that it is unable to metabolize sugars, but instead rely on amino acids that are eventually catabolized by the citric acid cycle during aerobic respiration. It survives on the remains of less halophilic organisms that have lysed due to the overwhelming amounts of salt in the environment. Outside of their natural environment, cells are cultured best in a complex medium. A minimal medium described for Halobacterium includes all but 5 of the 20 amino acids for growth.[4]
This microorganism has been studied extensively and has been shown to contain some of the usual features found in halophilic archaea, for example, an S-layer glycoprotein, ether-linked lipids, and purple membrane.[8] This purple membrane consists of the light-driven ion transporters bacteriorhodopsin and halorhodopsin, and the phototaxis receptors, sensory rhodopsins I and II.[4] In order to survive in low oxygen environments, Halobacterium sp. NRC-1 increases its production of Bacteriorhodopsin, which is a unique protein that can use light as an energy source, much like chlorophyll can in cyanobacteria and phototrophic eukaryotes. When the retinal in Bacteriorhodopsin absorbs light, it results in a series of conformational changes that translocates protons through the cell's membrane into the periplasmic space. This light driven proton pumping generates an electrochemical proton gradient which is then used to power the synthesis of ATP. This phototrophic capability is particularly useful to Halobacterium sp. NRC-1 as oxygen is not very soluble in concentrated salt solutions. In addition to its ability to use light as an energy source, it is also capable of anaerobic respiration using dimethyl sulfoxide (DMSO) and trimethylamine-N-oxide (TMAO) as terminal electron acceptors. Arginine fermentation can also be used for anaerobic energy production as well.[9]
Halobacterium sp. NRC-1 is also classified as an obligate halophilic microorganism which has adapted to be able to grow in conditions of extremely high salinity, up to 10 times that of seawater.[4] In order to survive under these conditions it maintains a very high concentration of salts internally in the form of KCl to enable it to remain isotonic to its preferred environment.[10] Halorhodopsin plays a very important role in helping to maintain the osmotic balance within the cell. This membrane protein acts as a light driven pump by transporting chloride and potassium ions into the cell. Halorhodopsin saves the organism a large amount of metabolic energy by using the energy of the yellow light that it captures to fuel the movement of these ions.[11]
Ecology
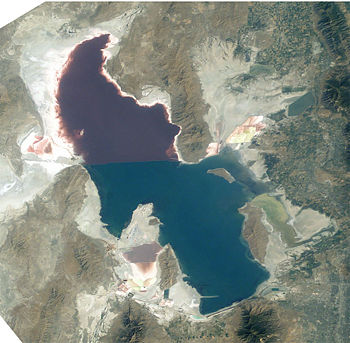
Halobacterium NRC-1 is one of many strains of halobacterium which thrive in extremely high salinity environments such as salt lakes, salt marshes and salt drying ponds. Their optimal temperature for reproduction is 42°C. Often these highly saline bodies of water will be tinted red or purple. It is the red/purple color of the bacteriorhodopsins that give the purple color you often see in these highly saline environments. Bacteriorhodopsin consists of a photosensitive protein pigment called retinal. This protein pigment is responsible for NRC-1's colorful properties. The more saline the environment the more colorful the water will be because halobacterium increase their production of bacteriorhodopsin in response to drops in oxygen, which is less soluble in saline solutions.
There are not many other organisms that can survive in these high salt environments, in fact one of its primary sources of food is the amino acids of other organisms which have lysed due to the high salt concentration in this environment. Brine shrimp are one of a few other organisms that can survive the high salt concentration, and they feed almost exclusively on the halobacteria. In addition to rhodopsin, Halobacterium NRC-1 produce carotenoids, which are red organic pigments that can also serve as antioxidants. The flamingo, whose pink color comes exclusively from the carotenoids in its diet (it doesn't have the ability to produce these pigments naturally) feed on the brine shrimp (who are capable of producing these pigments). These same brine shrimp have fed on the carotenoid producing halobacteria. So interestingly enough, these small organisms, the brine shrimp and the halobacteria, are the ones responsible for the flamingos beautiful pink coloring.
Application to Biotechnology
Halobacterium NRC-1 can easily be analyzed just by placing it in a hypotonic solution. Due to the higher salt concentration within the microorganism in comparison to its less salinic environment, water will flow into the cell following its concentration gradient, causing the cell to swell and undergo lysis. This explosion releases this microorganism's proteins, which then can be used by researchers for genetic analysis. Halobacterium sp. NRC-1 was one of the first Archaea to have its genome fully mapped and published. Since that time several more archaea have been successfully mapped and a few others partially mapped. This allows scientists to analyze the properties of halobacterium in silico to determine the activity of genes. Transformation tools have also been developed to create loss-of-function mutants which aid in research. Mutant strains of Halobacterium sp. NRC-1 are experimentally designed to carry inoperative knockout genes which alter this organism's normal cellular processes. Scientists can then infer the function of the mutated gene by comparing the physical and biochemical characteristics of the original Halobacterium sp. NRC-1 with the characteristics of the new mutant. These gene knockouts are making it possible to analyze this organism's ability to withstand extreme environmental conditions, including high levels of radiation, severe temperature changes, and fluctuations in oxygen levels. [7] The ability of this organism to be easily cultured along with these advanced research tools make Halobacterium sp. NRC-1 an ideal model for the testing of gene functions.
In addition, Halobacterium sp. NRC-1 is an ideal study tool because it is an extremophile, it exists in environments that are very unfriendly toward life. This makes it very useful for studying many biological questions especially those involved with adaptation and survival in extreme environments. In fact, much research is being done to investigate whether or not halobacterium could be potential candidates for extraterrestrial life, such as on Mars or Europa. Recent research has shown that Halobacterium sp. NRC-1 can not only survive at temperatures far below its optimal growth temperature, but continue to reproduce as well.[12]
Current Research
Protective effect of cations in extreme environments
The extremophillic properties and biological responses of Halobacteruim NRC-1 are currently being investigated to test the organism's survival potential in the harsh environmental conditions that exist on Mars. The recent discoveries that meterorites from Mars contain a mineral form of sodium chloride as well as the isolation of halophilic archaea from ancient rock salt has led scientists to believe that the existence of Halobacterium sp. NRC-1 in a Martian environment is quite possible. Halobacterium are known to be slightly thermophilic organisms, showing optimum growth at 40 degrees Celsius. In this experiment, cells of Halobacterium sp. NRC-1 were freeze dried and suspended in buffers containing specific cations, including Mg++,Ca++,or K+. Results showed that NRC-1 was still capable of slow, yet steady growth at the extremely low temperature of -15 degrees Celsius. All of the cations appeared to show protective qualities during the freeze-drying, with K+ being the most effective. The conditions of Martian environment include very low temperatures and low water availability. Scientists will continue to research NRC-1’s ability to survive in such inhospitable environments such as the one on Mars. Hopefully, these future findings will provide us some further insight in our search for life beyond our planet.[12]
Radiation resistance
Halobacterium sp. NRC-1 is known to be extremely radiation resistant due to protection from its pigments, the saline environment it thrives in, and its ability to repair its DNA after extensive damage. In this study the scientists wanted to find out if they could produce a mutant strain that would be even more resistant to ionizing radiation, and determine what genes and mechanisms were responsible for providing the increased resistance. To do this, they exposed cultures of H. NRC-1 to four cycles of irradiation with high doses of 18-20 MeV (mega-electron volt) electrons from a standard medical LINAC (particle accelerator), allowing the cultures to recover between doses. At the end of this process they were able to isolate two mutant strains (named LH5 and LH7a) with an LD50 (median lethal dose) of greater than 11 kGy (kilo-Grays) (standard measure of absorbed radiation dose) which makes them the most radiation resistant organism known, even more resistant than Deinococcus radiodurans (LD50 7.9 kGy). The wild-type H. NRC-1 was found to have an LD50 of 5.4 kGy while the mutant strains were 11.9 kGy for LH5 and 12.1 kGy for LH7a. Unlike most other microbes like D. radiodurans which show the most radiation resistance during their stationary phase, H. NRC-1 is most resistant when in its growth phase. Tests verified that this was true for the mutants as well. They were also able to rule out a slowdown in growth rate, which is one of the methods employed by D. radiodurans, as the reason for the increased resistance. To do this, they compared the doubling rate of the mutant strains with that of the wild-type H. NRC-1 and found it to be the same. They then used whole-genome transcriptome analysis to compare the mutant strains with the wild type H. NRC-1 to determine what had given the mutant strains their increased radiation resistance. They found significant up-regulation of an operon containing two single-stranded DNA-binding protein (RPA-replication protein A) genes, VNG2160 (rfa3) and VNG2162, plus a third gene, VNG2163, which is unknown. While the authors tests did not identify the actual role this operon plays in providing the increased resistance to radiation, they propose that it does facilitate the DNA repair machinery and/or protects the repair intermediates to maximize radiation resistance.[13]
Peptide delivery vehicle for biotechnology
Halobacterium sp. NRC-1 is also playing a significant role in the advancement of immunological biotechnology. Recent experimental findings show that recombinant gas vesicles from a mutant strain of Halobacterium sp. NRC-1 have the potential to serve as antigen display/delivery systems. DNA segments of an SIV (simian immunodeficiency virus) gene were inserted into a specific site on a GvpC gene (gas vesicle protein C) in the Halobacterium. The gene was successfully taken up by the microorganism and recombination had been accomplished. Subsequently, the gas vesicles began to express the new recombinant/pathogenic proteins at their surface. Mice were then immunized with these antigen presenting SIV recombinant gas vesicles. Antibody production was monitored, and an increased level of humoral response was observed. After a 12 week period, a booster shot was given. 43 weeks after the booster shot was administered, an elevated number of antibodies still remained and was recorded. Research showed that this increased production of antibodies was long lived even in the absence of an adjuvant, and immunologic memory had been proven. These results indicate that the gas vesicles of Halobacterium sp. NRC-1 show great potential in their possible roles as immunizing agents.[14]
References
- ↑ Woese, C R (1994-03). "There must be a prokaryote somewhere: microbiology's search for itself". Microbiological Reviews 58 (1): 1-9. ISSN 0146-0749. Retrieved on 2009-04-28.
- ↑ Waggoner, Ben; Brian R. Speer (20 April 2001). Introduction to the Archaea: Life's extremists…. University of California Museum of Paleontology. Retrieved on 8 November 2013.
- ↑ McCready, Shirley; Jochen Muller, Ivan Boubriak, Brian Berquist, Wooi Ng, Shiladitya DasSarma (2005). "UV irradiation induces homologous recombination genes in the model archaeon, Halobacterium sp. NRC-1". Saline Systems 1 (1): 3. DOI:10.1186/1746-1448-1-3. ISSN 1746-1448. Retrieved on 2009-04-18. Research Blogging.
- ↑ 4.0 4.1 4.2 4.3 4.4 Ng, Wailap Victor, et al. (2000-10-24). "Genome sequence of Halobacterium species NRC-1". Proceedings of the National Academy of Sciences of the United States of America 97 (22): 12176-12181. DOI:- 97 VL - 97. Retrieved on 2009-04-18. - 97 Research Blogging.
- ↑ Kottemann, Molly; Adrienne Kish, Chika Iloanusi, Sarah Bjork, Jocelyne DiRuggiero (2005-06-01). "Physiological responses of the halophilic archaeon Halobacterium sp. strain NRC1 to desiccation and gamma irradiation". Extremophiles 9 (3): 219-227. DOI:10.1007/s00792-005-0437-4. Retrieved on 2009-04-28. Research Blogging.
- ↑ 6.0 6.1 6.2 DasSarma, Shiladitya; Brian Berquist, James Coker, Priya DasSarma, Jochen Muller (2006). "Post-genomics of the model haloarchaeon Halobacterium sp. NRC-1". Saline Systems 2 (1): 3. DOI:10.1186/1746-1448-2-3. ISSN 1746-1448. Retrieved on 2009-04-18. Research Blogging.
- ↑ 7.0 7.1 Soppa, Jorg (2006-03-01). "From genomes to function: haloarchaea as model organisms". Microbiology 152 (3): 585-590. DOI:10.1099/mic.0.28504-0. Retrieved on 2009-04-18. Research Blogging.
- ↑ Kennedy, S P; W V Ng, S L Salzberg, L Hood, S DasSarma (2001-10). "Understanding the adaptation of Halobacterium species NRC-1 to its extreme environment through computational analysis of its genome sequence". Genome Research 11 (10): 1641-50. DOI:10.1101/gr.190201. ISSN 1088-9051. Retrieved on 2009-04-18. Research Blogging.
- ↑ Müller, Jochen A.; Shiladitya DasSarma (2005-03). "Genomic Analysis of Anaerobic Respiration in the Archaeon Halobacterium sp. Strain NRC-1: Dimethyl Sulfoxide and Trimethylamine N-Oxide as Terminal Electron Acceptors". Journal of Bacteriology 187 (5): 1659–1667. DOI:10.1128/JB.187.5.1659-1667.2005. Retrieved on 2009-04-18. Research Blogging.
- ↑ Coker, James; Priya DasSarma, Jeffrey Kumar, Jochen Muller, Shiladitya DasSarma (2007). "Transcriptional profiling of the model Archaeon Halobacterium sp. NRC-1: responses to changes in salinity and temperature". Saline Systems 3 (1): 6. DOI:10.1186/1746-1448-3-6. ISSN 1746-1448. Retrieved on 2009-04-18. Research Blogging.
- ↑ Oesterhelt, D (1998-08). "The structure and mechanism of the family of retinal proteins from halophilic archaea". Current Opinion in Structural Biology 8 (4): 489-500. ISSN 0959-440X. Retrieved on 2009-04-26.
- ↑ 12.0 12.1 Weidler, Gerhard (2004-03-01), Survival and growth of Halobacterium sp. NRC-1 following incubation at -15°C, freezing or freeze-drying, and the protective effect of cations, Third European Workshop on Exo-Astrobiology, vol. 545, at 311-312. Retrieved on 2009-04-18
- ↑ Linda C. DeVeaux; Jochen A. Müller, Jonathon Smith, Jill Petrisko, Douglas P. Wells, Shiladitya DasSarma (2007). "Extremely Radiation-Resistant Mutants of a Halophilic Archaeon with Increased Single-Stranded DNA-Binding Protein (RPA) Gene Expression". Radiation Research 168 (4): 507-514. DOI:10.1667/RR0935.1. Retrieved on 2009-05-10. Research Blogging.
- ↑ Sremac, Marinko; Elizabeth S Stuart (2008). "Recombinant gas vesicles from Halobacterium sp. displaying SIV peptides demonstrate biotechnology potential as a pathogen peptide delivery vehicle". BMC Biotechnology 8: 9. DOI:10.1186/1472-6750-8-9. Retrieved on 2009-04-30. Research Blogging.
Previous Winners
 Animal: A multicellular organism that feeds on other organisms, and is distinguished from plants, fungi, and unicellular organisms. [e] (May 28)
Animal: A multicellular organism that feeds on other organisms, and is distinguished from plants, fungi, and unicellular organisms. [e] (May 28) Coal: a combustible, black rock formed after millions of years of heat and pressure were applied to the decayed remains of plants and organic matter in what were then swamps. [e] (May 21)
Coal: a combustible, black rock formed after millions of years of heat and pressure were applied to the decayed remains of plants and organic matter in what were then swamps. [e] (May 21) Johannes Diderik van der Waals: (1837 – 1923) Dutch scientist, proposed the van der Waals equation of state for gases. [e] (May 7)
Johannes Diderik van der Waals: (1837 – 1923) Dutch scientist, proposed the van der Waals equation of state for gases. [e] (May 7) Scientific method: The concept of systematic inquiry based on hypotheses and their testing in light of empirical evidence. [e] (Apr 14)
Scientific method: The concept of systematic inquiry based on hypotheses and their testing in light of empirical evidence. [e] (Apr 14) Korematsu v. United States: A U.S. Supreme Court case, in which the internment of Japanese-Americans was deemed constitutional due to military necessity [e] (Apr 7)
Korematsu v. United States: A U.S. Supreme Court case, in which the internment of Japanese-Americans was deemed constitutional due to military necessity [e] (Apr 7) Orchid: Any plant classified under Orchidaceae, one of the largest plant families and the largest among Monocotyledons. [e] (Mar 31)
Orchid: Any plant classified under Orchidaceae, one of the largest plant families and the largest among Monocotyledons. [e] (Mar 31) Oliver Cromwell: (1599-1658) English soldier, statesman, and leader of the Puritan revolution, nicknamed "Old Ironsides". [e] (Mar 24)
Oliver Cromwell: (1599-1658) English soldier, statesman, and leader of the Puritan revolution, nicknamed "Old Ironsides". [e] (Mar 24) Wisconsin v. Yoder: 1972 U.S. Supreme Court decision in which it was held that the constitutional rights of the Amish, under the "free exercise of religion" clause, were violated by the state's compulsory school attendance law. [e] (Mar 17)
Wisconsin v. Yoder: 1972 U.S. Supreme Court decision in which it was held that the constitutional rights of the Amish, under the "free exercise of religion" clause, were violated by the state's compulsory school attendance law. [e] (Mar 17) Conventional coal-fired power plant: power plant that burns coal in a steam generator to produce high pressure steam, which goes to steam turbines that generate electricity. [e] (Mar 10)
Conventional coal-fired power plant: power plant that burns coal in a steam generator to produce high pressure steam, which goes to steam turbines that generate electricity. [e] (Mar 10) Battle of the Ia Drang: First divisional-scale battle involving helicopter-borne air assault troops, with U.S. forces against those of North Vietnam [e] (Mar 3)
Battle of the Ia Drang: First divisional-scale battle involving helicopter-borne air assault troops, with U.S. forces against those of North Vietnam [e] (Mar 3) Ether (physics): Medium that can carry electromagnetic waves (obsolete) [e] (Feb 24)
Ether (physics): Medium that can carry electromagnetic waves (obsolete) [e] (Feb 24) Large-scale trickle filters: One of the processes by which biodegradable substances in wastewaters are biochemically oxidized. [e] (11 Feb)
Large-scale trickle filters: One of the processes by which biodegradable substances in wastewaters are biochemically oxidized. [e] (11 Feb) Homeopathy: System of alternative medicine involving administration of highly diluted substances with the intention to stimulate the body's natural healing processes, not considered proven by mainstream science. [e] (28 Jan)
Homeopathy: System of alternative medicine involving administration of highly diluted substances with the intention to stimulate the body's natural healing processes, not considered proven by mainstream science. [e] (28 Jan) Microeconomics: A branch of economics that deals with transactions between suppliers and consumers, acting individually or in groups. [e] (14 Jan)
Microeconomics: A branch of economics that deals with transactions between suppliers and consumers, acting individually or in groups. [e] (14 Jan) Speech Recognition: The ability to recognize and understand human speech, especially when done by computers. [e] (26 Nov)
Speech Recognition: The ability to recognize and understand human speech, especially when done by computers. [e] (26 Nov) Mashup: A data visualization created by combining data with multiple computer applications. [e] (19 Nov)
Mashup: A data visualization created by combining data with multiple computer applications. [e] (19 Nov) Tux: The name of the penguin, official logo and cartoon mascot for the Linux computer operating system. [e] (14 Oct)
Tux: The name of the penguin, official logo and cartoon mascot for the Linux computer operating system. [e] (14 Oct) Hydrogen bond: A non-covalent and non-ionic chemical bond involving a hydrogen atom and either Fluorine, Nitrogen, or Oxygen. [e] (7 Oct)
Hydrogen bond: A non-covalent and non-ionic chemical bond involving a hydrogen atom and either Fluorine, Nitrogen, or Oxygen. [e] (7 Oct) Lead: Chemical element number 82, a corrosion-resistant, dense, ductile heavy metal known to cause neurological problems. [e] (1 Sept)
Lead: Chemical element number 82, a corrosion-resistant, dense, ductile heavy metal known to cause neurological problems. [e] (1 Sept) DNA: A macromolecule — chemically, a nucleic acid — that stores genetic information. [e] (8 July)
DNA: A macromolecule — chemically, a nucleic acid — that stores genetic information. [e] (8 July) Augustin-Louis_Cauchy: (1789 – 1857) prominent French mathematician, one of the pioneers of rigor in mathematics and complex analysis. [e] (1 July)
Augustin-Louis_Cauchy: (1789 – 1857) prominent French mathematician, one of the pioneers of rigor in mathematics and complex analysis. [e] (1 July) Vasco da Gama: Portuguese explorer who established a sea route from Europe to India. [e] (24 June)
Vasco da Gama: Portuguese explorer who established a sea route from Europe to India. [e] (24 June) Phosphorus: Add brief definition or description (17 June)
Phosphorus: Add brief definition or description (17 June) Crystal Palace: Add brief definition or description (10 June)
Crystal Palace: Add brief definition or description (10 June) Gross Domestic Product: Add brief definition or description (3 June)
Gross Domestic Product: Add brief definition or description (3 June) RNA interference: Add brief definition or description (27 May)
RNA interference: Add brief definition or description (27 May) Latino history: Add brief definition or description (20 May)
Latino history: Add brief definition or description (20 May) Navy Grog: Add brief definition or description (13 May)
Navy Grog: Add brief definition or description (13 May) Systems biology: Add brief definition or description (6 May)
Systems biology: Add brief definition or description (6 May) Steroid: Add brief definition or description (22 Apr)
Steroid: Add brief definition or description (22 Apr) Lebanon: Add brief definition or description (15 Apr)
Lebanon: Add brief definition or description (15 Apr) Wheat: Add brief definition or description (7 Apr)
Wheat: Add brief definition or description (7 Apr) Benjamin Franklin: Add brief definition or description (1 Apr)
Benjamin Franklin: Add brief definition or description (1 Apr) Coherer: Add brief definition or description (25 Mar)
Coherer: Add brief definition or description (25 Mar) U.S. Civil War: Add brief definition or description (18 Mar)
U.S. Civil War: Add brief definition or description (18 Mar) Life: Add brief definition or description (11 Mar)
Life: Add brief definition or description (11 Mar) Petroleum refining processes: Add brief definition or description (4 Mar)
Petroleum refining processes: Add brief definition or description (4 Mar) Shirley Chisholm: Add brief definition or description (20 Feb)
Shirley Chisholm: Add brief definition or description (20 Feb) Telephone Newspaper: Add brief definition or description (4 Feb)
Telephone Newspaper: Add brief definition or description (4 Feb) Wristwatch: Add brief definition or description (28 Jan)
Wristwatch: Add brief definition or description (28 Jan) Korean War of 1592-1598: Add brief definition or description (21 Jan)
Korean War of 1592-1598: Add brief definition or description (21 Jan) Andrew Carnegie: Add brief definition or description (11 January 2008)
Andrew Carnegie: Add brief definition or description (11 January 2008)- Bowling: Add brief definition or description (31 December 2007)
 Architecture: Add brief definition or description (December 6)
Architecture: Add brief definition or description (December 6) Civil society: Add brief definition or description November 29
Civil society: Add brief definition or description November 29 Joan of Arc: Add brief definition or description (November 22)
Joan of Arc: Add brief definition or description (November 22) Chemistry: Add brief definition or description (November 15)
Chemistry: Add brief definition or description (November 15) Albert Gallatin: Add brief definition or description (November 8)
Albert Gallatin: Add brief definition or description (November 8) Prime number: Add brief definition or description (November 1)
Prime number: Add brief definition or description (November 1) Tennis: Add brief definition or description (October 25)
Tennis: Add brief definition or description (October 25) Rottweiler: Add brief definition or description (October 18)
Rottweiler: Add brief definition or description (October 18) Theodor Lohmann: Add brief definition or description (October 9)
Theodor Lohmann: Add brief definition or description (October 9) William Shakespeare: Add brief definition or description (October 2)
William Shakespeare: Add brief definition or description (October 2) Edward I: Add brief definition or description (September 25)
Edward I: Add brief definition or description (September 25) El Tío: Add brief definition or description (September 18)
El Tío: Add brief definition or description (September 18) Scotland Yard: Add brief definition or description (September 11)
Scotland Yard: Add brief definition or description (September 11) Kilt: Add brief definition or description (September 4)
Kilt: Add brief definition or description (September 4) U.S. Electoral College: Add brief definition or description (August 28)
U.S. Electoral College: Add brief definition or description (August 28) Butler: Add brief definition or description (August 21)
Butler: Add brief definition or description (August 21) Tony Blair: Add brief definition or description (August 14)
Tony Blair: Add brief definition or description (August 14) Northwest Passage: Add brief definition or description (August 7)
Northwest Passage: Add brief definition or description (August 7) Literature: Add brief definition or description (July 31)
Literature: Add brief definition or description (July 31) Biology: Add brief definition or description (July 25)
Biology: Add brief definition or description (July 25)
Rules and Procedure
Rules
- The article's status must be 0 or 1, i.e., only "Advanced Articles" may be nominated.
- Any Citizen may nominate an article.
- No Citizen may have nominated more than one article listed under "current nominees" at a time.
- The article's nominator is indicated simply by the first name in the list of votes (see below).
- At least for now--while the project is still small--you may nominate and vote for articles of which you are a main author.
- An article can be Article of the Week only once every six months. Nominated articles that have won top honors should be removed from the list.
- Comments on nominations should be made on the article's talk page.
- The list of nominees should be kept below 20, or thereabouts. Articles with very few supporters and which have not gained any new supporters in the last two weeks or so may be deleted to make room for new nominees.
- Any editor may entirely cancel the nomination of any unapproved article in his or her area of expertise if, for example, it contains obvious and embarrassing problems.
Nomination
- Before nominating an article, you must make the changes listed at Template:Featured Article Candidate.
- Then, you can add the article to the list above using the {{Featured Article Candidate}} template.
- Add your article in the correct position in the list according to the rules below.
Voting
- To vote, add your name and date in the Supporters column next to an article title, after other supporters for that article, by signing <br />~~~~. (The date is necessary so that we can determine when the last vote was added.)
- Add your name in the Specialist supporters column only if you are an editor who is an expert about the topic in question. Your vote will be counted as three.
- You may vote for as many articles as you wish, and each vote counts separately, but you can only nominate one at a time; see above. You could, theoretically, vote for every nominated article on the page, but this would be pointless.
Ranking
- The list of articles is sorted by number of votes first, then alphabetically.
- Admins should make sure that the votes are correctly tallied, but anyone may do this. Note that "Specialist Votes" are worth 3 points.
Updating
- Each Thursday, one of the admins listed below should move the winning article to the Current Winner section of this page, announces the winner on Citizendium-L and updates the "previous winning articles" section accordingly.
- The winning article will be the article at the top of the list (ie the one with the most votes).
- In the event of two or more having the same number of votes :
- The article with the most specialist supporters is used. Should this fail to produce a winner, the article appearing first by English alphabetical order is used.
- The remaining winning articles are guaranteed this position in the following weeks, again in alphabetical order. No further voting would take place on these, which remain at the top of the table with notices to that effect. Further nominations and voting take place to determine future winning articles for the following weeks.
- The article with the most specialist supporters is used. Should this fail to produce a winner, the article appearing first by English alphabetical order is used.
Administrators
These are people who have volunteered to run this program. Their duties are (1) to ensure that this page remains "clean," e.g., as a given article garners more votes, its tally is accurately represented and it moves up the list, and (2) to place the winning article on the front page on a weekly basis. To become an administrator, you need not apply anywhere. Simply add your name below. Administrator duties are open to editors and authors alike.
See Also





























