Colorectal cancer: Difference between revisions
imported>Robert Badgett |
imported>Robert Badgett (→Practice guidelines: new guideline and organized) |
||
| Line 49: | Line 49: | ||
===Practice guidelines=== | ===Practice guidelines=== | ||
====US Preventive Services Task Force==== | |||
A [[clinical practice guideline]] by the [[US Preventive Services Task Force]] has addressed colorectal cancer:<ref name="pmid18838716">{{cite journal |author= |title=Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement |journal=Annals of Internal Medicine |volume= |issue= |pages= |year=2008 |month=October |pmid=18838716 |doi= |url=http://www.annals.org/cgi/content/full/0000605-200811040-00243v1 |issn=}}</ref> | A [[clinical practice guideline]] by the [[US Preventive Services Task Force]] has addressed colorectal cancer:<ref name="pmid18838716">{{cite journal |author= |title=Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement |journal=Annals of Internal Medicine |volume= |issue= |pages= |year=2008 |month=October |pmid=18838716 |doi= |url=http://www.annals.org/cgi/content/full/0000605-200811040-00243v1 |issn=}}</ref> | ||
* "recommends screening for colorectal cancer using fecal occult blood testing, sigmoidoscopy, or colonoscopy in adults, beginning at age 50 years and continuing until age 75 years." | * "recommends screening for colorectal cancer using fecal occult blood testing, sigmoidoscopy, or colonoscopy in adults, beginning at age 50 years and continuing until age 75 years." | ||
| Line 58: | Line 59: | ||
* "the evidence is insufficient to assess the benefits and harms of computed tomographic colonography and fecal DNA testing (a subsequent study found that DNA was more [[sensitivity and specificity|sensitive]] but less [[sensitivity and specificity|specific]]<ref name="pmid18838724">{{cite journal |author=Ahlquist DA, Sargent DJ, Loprinzi CL, ''et al'' |title=Stool DNA and occult blood testing for screen detection of colorectal neoplasia |journal=Ann. Intern. Med. |volume=149 |issue=7 |pages=441–50, W81 |year=2008 |month=October |pmid=18838724 |doi= |url=http://www.annals.org/cgi/pmidlookup?view=long&pmid=18838724 |issn=}}</ref>)" | * "the evidence is insufficient to assess the benefits and harms of computed tomographic colonography and fecal DNA testing (a subsequent study found that DNA was more [[sensitivity and specificity|sensitive]] but less [[sensitivity and specificity|specific]]<ref name="pmid18838724">{{cite journal |author=Ahlquist DA, Sargent DJ, Loprinzi CL, ''et al'' |title=Stool DNA and occult blood testing for screen detection of colorectal neoplasia |journal=Ann. Intern. Med. |volume=149 |issue=7 |pages=441–50, W81 |year=2008 |month=October |pmid=18838724 |doi= |url=http://www.annals.org/cgi/pmidlookup?view=long&pmid=18838724 |issn=}}</ref>)" | ||
====American College of Gastroenterology==== | |||
[[Clinical practice guideline]] published in 2009 by the American College of Gastroenterology recommends:<ref name="pmid19240699">{{cite journal| author=Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM et al.| title=American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. | journal=Am J Gastroenterol | year= 2009 | volume= 104 | issue= 3 | pages= 739-50 | pmid=19240699 | doi=10.1038/ajg.2009.104 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19240699 }} [http://www.acg.gi.org/physicians/pdfs/CCSJournalPublicationFebruary2009.pdf ACG website]</ref> | |||
* "Cancer prevention tests should be offered fi rst. The preferred CRC prevention test is colonoscopy every 10 years, beginning at age 50. (Grade 1 B) Screening should begin at age 45 years in African Americans (Grade 2 C)" | |||
* "Cancer detection test. This test should be offered to patients who decline colonoscopy or another cancer prevention test. The preferred cancer detection test is annual FIT for blood (Grade 1 B)" | |||
''Notes'': | |||
Grade 2C recommendation reflects "2C/Weak recommendation, low-quality or very low-quality evidence" | |||
====American Cancer Society==== | |||
''For screening'', a [[clinical practice guideline]] jointly published in 2007 by the [[American Cancer Society]] and other groups recommends one of:<ref>Levin, B., Lieberman, D. A., McFarland, B., Smith, R. A., Brooks, D., Andrews, K. S., et al. (2008). [http://caonline.amcancersoc.org/cgi/content/full/CA.2007.0018v1 Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-society Task Force on Colorectal Cancer, and the American College of Radiology]. CA Cancer J Clin, CA.2007.0018. {{doi|10.3322/CA.2007.0018}}.</ref> | |||
* Flexible sigmoidoscopy every 5 years | * Flexible sigmoidoscopy every 5 years | ||
* Barium enema every 5 years | * Barium enema every 5 years | ||
| Line 64: | Line 73: | ||
* Colonoscopy every 10 years | * Colonoscopy every 10 years | ||
When polyps are found, a [[clinical practice guideline]] jointly | ''When polyps are found'', a [[clinical practice guideline]] jointly published in 2006 by the [[American Cancer Society]] and other groups states:<ref name="pmid16697750">{{cite journal |author=Winawer SJ, Zauber AG, Fletcher RH, ''et al'' |title=Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society |journal=Gastroenterology |volume=130 |issue=6 |pages=1872–85 |year=2006 |month=May |pmid=16697750 |doi=10.1053/j.gastro.2006.03.012 |url=http://www.gastrojournal.org/article/S0016-5085(06)00561-0/fulltext |issn=}}</ref> | ||
* High risk polyps are 1) 3 or more synchronous adenomas, 2) adenomas ≥1 cm in diameter, or 3) villous histology or high-grade dysplasia. | * High risk polyps are 1) 3 or more synchronous adenomas, 2) adenomas ≥1 cm in diameter, or 3) villous histology or high-grade dysplasia. | ||
* High risk polyps should have follow-up colonoscopy in 3 years | * High risk polyps should have follow-up colonoscopy in 3 years | ||
Revision as of 10:48, 22 August 2011
Pathophysiology
Colorectal cancer probably arises from colorectal polyps.[1] Adenomatous polyps convert to cancers at a rate of about 1% per year.[2]
Treatment
Colorectal cancer treatment information from the National Cancer Institute's Physician Data Query
Medications
Aspirin
Aspirin may reduce mortality among patients whose tumor overexpress the enzyme cyclooxygenase 2 according to a cohort study.[3] Cyclooxygenase 2 is expressed by most colorectal cancers and is associated with reduced survival.[4]
Cetuximab
Cetuximab, an IgG1 chimeric monoclonal antibody against epidermal growth factor receptor, may help according to a randomized controlled trial.[5]
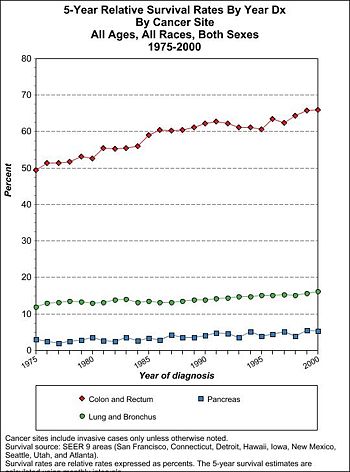
Prognosis
Staging information
Colorectal cancer staging information from the National Cancer Institute's Physician Data Query
| Stage | Five-year survival rate (%) |
|---|---|
| Stage I (T1-2N0) | 93 |
| Stage IIA (T3N0) | 85 |
| Stage IIB (T4N0) | 72 |
| Stage IIIA (T1-2 N1) | 83 |
| Stage IIIB (T3-4 N1) | 64 |
| Stage IIIC (N2) | 44 |
| Stage IV | 8 |
Screening
In the United States, both underuse and overuse of screening occur.[7]
Practice guidelines
US Preventive Services Task Force
A clinical practice guideline by the US Preventive Services Task Force has addressed colorectal cancer:[8]
- "recommends screening for colorectal cancer using fecal occult blood testing, sigmoidoscopy, or colonoscopy in adults, beginning at age 50 years and continuing until age 75 years."
- "recommends against routine screening for colorectal cancer in adults 76 to 85 years of age. There may be considerations that support colorectal cancer screening in an individual patient."
- "recommends against screening for colorectal cancer in adults older than age 85 years"
- "the evidence is insufficient to assess the benefits and harms of computed tomographic colonography and fecal DNA testing (a subsequent study found that DNA was more sensitive but less specific[9])"
American College of Gastroenterology
Clinical practice guideline published in 2009 by the American College of Gastroenterology recommends:[10]
- "Cancer prevention tests should be offered fi rst. The preferred CRC prevention test is colonoscopy every 10 years, beginning at age 50. (Grade 1 B) Screening should begin at age 45 years in African Americans (Grade 2 C)"
- "Cancer detection test. This test should be offered to patients who decline colonoscopy or another cancer prevention test. The preferred cancer detection test is annual FIT for blood (Grade 1 B)"
Notes: Grade 2C recommendation reflects "2C/Weak recommendation, low-quality or very low-quality evidence"
American Cancer Society
For screening, a clinical practice guideline jointly published in 2007 by the American Cancer Society and other groups recommends one of:[11]
- Flexible sigmoidoscopy every 5 years
- Barium enema every 5 years
- Virtual colonography (a noninvasive test based on computed tomography) every 5 years
- Colonoscopy every 10 years
When polyps are found, a clinical practice guideline jointly published in 2006 by the American Cancer Society and other groups states:[12]
- High risk polyps are 1) 3 or more synchronous adenomas, 2) adenomas ≥1 cm in diameter, or 3) villous histology or high-grade dysplasia.
- High risk polyps should have follow-up colonoscopy in 3 years
- Low risk polyps should have repeat colonoscopy in 5 to 10 years
- If no adenomas are found, follow-up evaluation should be at 10 years
Evidence
A cost-benefit analysis[13] and a meta-analysis[14] have reviewed studies of fecal testing and colonic imaging. Immunochemical fecal occult blood (I-FOBT) tests may be the most effective.[13][15][16]
A validation of guidelines found:[17]
- High risk adenomas - 9% of an advanced adenoma at 4 years of follow-up.
- Low risk adenomas - 5% of an advanced adenoma at 4 years of follow-up.
Fecal testing
Feces can be tested for occult blood by either:
- Chemical reaction with guaiac
- Immumohistochemistry test for components of blood such as hemoglobin or haptoglobin. The method may be the most effective[13][15][16] and also may be improved if patients are taking low dose aspirin to prevent vascular disease.[18]
- DNA
Visualization
| Procedure | Study | Benefit | Number needed to screen (assuming control rate of 1%) |
|---|---|---|---|
| Fecal occult blood annually | Minnesota Colon Cancer Control Study[20] Randomized controlled trial 46,551 patients for 13 years |
Colorectal cancer death: Relative risk ratio 0.67 Relative risk reduction 33% |
305 |
| Double-contrast barium enema | Ontario Cancer Registry[21] Cohort study 13,849 patients who had a DCBE 36 months prior to the diagnosis of CRC |
Colorectal cancer incidence: False negative rate (1-Sensitivity) 22% Sensitivity 78% |
142 |
| Sigmoidoscopy | SCORE trial[19] Randomized controlled trial 34,272 subjects patients for 10 years |
Colorectal cancer death: Relative risk ratio 0.78 (insig) |
1025 |
| Kaiser Permanente[23] Case-control study 261 case patients and 868 control patients for 10 years |
Colorectal cancer death: Odds ratio 0.41 |
170 | |
| Telemark Polyp Study I[24] Cohort study 400 case patients and 399 controls for 7 to 11 years |
Colorectal cancer incidence: Relative risk ratio 0.2 Relative risk reduction 80% |
125 | |
| Colonoscopy | National Polyp Study[25] Cohort study 1418 patients for 5.8 years |
Colorectal cancer incidence: Relative risk ratio 0.1 Relative risk reduction 90% |
111 |
| Ontario Cancer Registry[26] Case-control study 10,292 case patients and 51,460 controls for 7.8 years |
Colorectal cancer death: Odds ratio 0.69 |
325 |
Visualization may be less effective in the right colon.[27][26]
Capsule endoscopy
Capsule endoscopy is less accurate than optical endoscopy.[28]
Prevention
Aspirin chemoprophylaxis
A clinical practice guideline by the U.S. Preventive Services Task Force (USPSTF) recommended against taking aspirin (grade D recommendation).[29] The Task Force acknowledged that aspirin may reduce the incidence of colorectal cancer, but "concluded that harms outweigh the benefits of aspirin and NSAID use for the prevention of colorectal cancer". Long-term doses over 81 mg per day may increase bleeding events.[30]
Subsequent meta-analyses conclude:
- "300 mg or more of aspirin a day for about 5 years is effective in primary prevention of colorectal cancer in randomised controlled trials, with a latency of about 10 years".[31]
- "Aspirin is effective for the prevention of colorectal adenomas in individuals with a history of these lesions."[32] The number needed to treat was about 33.
Aspirin and celecoxib may help individuals with an increased risk of CRC according to the NIHR Health Technology Assessment programme (UK).[33]
Calcium
A meta-analysis by the Cochrane Collaboration of randomized controlled trials published through 2002 concluded "Although the evidence from two RCTs suggests that calcium supplementation might contribute to a moderate degree to the prevention of colorectal adenomatous polyps, this does not constitute sufficient evidence to recommend the general use of calcium supplements to prevent colorectal cancer.".[34] Subsequently, one randomized controlled trial by the Women's Health Initiative (WHI) reported negative results.[35] A second randomized controlled trial reported reduction in all cancers, but had insufficient colorectal cancers for analysis.[36]
References
- ↑ Levine JS, Ahnen DJ (December 2006). "Clinical practice. Adenomatous polyps of the colon". N. Engl. J. Med. 355 (24): 2551–7. DOI:10.1056/NEJMcp063038. PMID 17167138. Research Blogging.
- ↑ Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL (November 1987). "Natural history of untreated colonic polyps". Gastroenterology 93 (5): 1009–13. PMID 3653628. [e]
- ↑ Chan AT, Ogino S, Fuchs CS (2009). "Aspirin use and survival after diagnosis of colorectal cancer.". JAMA 302 (6): 649-58. DOI:10.1001/jama.2009.1112. PMID 19671906. Research Blogging.
- ↑ Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K (2004). "Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer.". Clin Cancer Res 10 (24): 8465-71. DOI:10.1158/1078-0432.CCR-04-0653. PMID 15623626. Research Blogging.
- ↑ Jonker DJ, O'Callaghan CJ, Karapetis CS, et al (2007). "Cetuximab for the treatment of colorectal cancer". N. Engl. J. Med. 357 (20): 2040–8. DOI:10.1056/NEJMoa071834. PMID 18003960. Research Blogging.
- ↑ O'Connell JB, Maggard MA, Ko CY (2004). "Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging.". J Natl Cancer Inst 96 (19): 1420-5. DOI:10.1093/jnci/djh275. PMID 15467030. Research Blogging.
- ↑ Walter LC, Lindquist K, Nugent S, et al (April 2009). "Impact of age and comorbidity on colorectal cancer screening among older veterans". Ann. Intern. Med. 150 (7): 465–73. PMID 19349631. [e]
- ↑ (October 2008) "Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement". Annals of Internal Medicine. PMID 18838716. [e]
- ↑ Ahlquist DA, Sargent DJ, Loprinzi CL, et al (October 2008). "Stool DNA and occult blood testing for screen detection of colorectal neoplasia". Ann. Intern. Med. 149 (7): 441–50, W81. PMID 18838724. [e]
- ↑ Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM et al. (2009). "American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected."]. Am J Gastroenterol 104 (3): 739-50. DOI:10.1038/ajg.2009.104. PMID 19240699. Research Blogging. ACG website
- ↑ Levin, B., Lieberman, D. A., McFarland, B., Smith, R. A., Brooks, D., Andrews, K. S., et al. (2008). Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin, CA.2007.0018. DOI:10.3322/CA.2007.0018.
- ↑ Winawer SJ, Zauber AG, Fletcher RH, et al (May 2006). "Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society". Gastroenterology 130 (6): 1872–85. DOI:10.1053/j.gastro.2006.03.012. PMID 16697750. Research Blogging.
- ↑ 13.0 13.1 13.2 Heitman SJ, Hilsden RJ, Au F, Dowden S, Manns BJ, 2010 Colorectal Cancer Screening for Average-Risk North Americans: An Economic Evaluation. PLoS Med 7(11): e1000370. DOI:10.1371/journal.pmed.1000370
- ↑ Whitlock EP, Lin JS, Liles E, Beil TL, Fu R (November 2008). "Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force". Ann. Intern. Med. 149 (9): 638–58. PMID 18838718. [e]
- ↑ 15.0 15.1 Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E et al. (2007). "A quantitative immunochemical fecal occult blood test for colorectal neoplasia.". Ann Intern Med 146 (4): 244-55. PMID 17310048. [e]
- ↑ 16.0 16.1 Jellema P, van der Windt DA, Bruinvels DJ, Mallen CD, van Weyenberg SJ, Mulder CJ et al. (2010). "Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis.". BMJ 340: c1269. DOI:10.1136/bmj.c1269. PMID 20360221. PMC PMC2848719. Research Blogging.
- ↑ Laiyemo AO, Murphy G, Albert PS, et al (March 2008). "Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years". Ann. Intern. Med. 148 (6): 419–26. PMID 18347350. [e]
- ↑ Brenner, Hermann; Sha Tao, Ulrike Haug (2010-12-08). "Low-Dose Aspirin Use and Performance of Immunochemical Fecal Occult Blood Tests". JAMA: The Journal of the American Medical Association 304 (22): 2513 -2520. DOI:10.1001/jama.2010.1773. Retrieved on 2010-12-08. Research Blogging.
- ↑ 19.0 19.1 Segnan, N (2011 [last update]). Once-Only Sigmoidoscopy in Colorectal Cancer Screening: Follow-up Findings of the Italian Randomized Controlled Trial—SCORE. jnci.oxfordjournals.org. Retrieved on August 19, 2011.
- ↑ 20.0 20.1 Mandel JS, Bond JH, Church TR, et al (May 1993). "Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study". N. Engl. J. Med. 328 (19): 1365–71. PMID 8474513. [e]
- ↑ 21.0 21.1 Toma J, Paszat LF, Gunraj N, Rabeneck L (December 2008). "Rates of new or missed colorectal cancer after barium enema and their risk factors: a population-based study". Am. J. Gastroenterol. 103 (12): 3142–8. DOI:10.1111/j.1572-0241.2008.02199.x. PMID 18853981. Research Blogging.
- ↑ Ransohoff DF, Lang CA (March 1993). "Sigmoidoscopic screening in the 1990s". JAMA 269 (10): 1278–81. PMID 8437306. [e]
- ↑ 23.0 23.1 Selby JV, Friedman GD, Quesenberry CP, Weiss NS (March 1992). "A case-control study of screening sigmoidoscopy and mortality from colorectal cancer". N. Engl. J. Med. 326 (10): 653–7. PMID 1736103. [e]
- ↑ 24.0 24.1 Thiis-Evensen E, Hoff GS, Sauar J, Langmark F, Majak BM, Vatn MH (April 1999). "Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I". Scand. J. Gastroenterol. 34 (4): 414–20. PMID 10365903. [e]
- ↑ 25.0 25.1 Winawer SJ, Zauber AG, Ho MN, et al (December 1993). "Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup". N. Engl. J. Med. 329 (27): 1977–81. PMID 8247072. [e]
- ↑ 26.0 26.1 26.2 Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L (January 2009). "Association of colonoscopy and death from colorectal cancer". Ann. Intern. Med. 150 (1): 1–8. PMID 19075198. [e]
Cite error: Invalid
<ref>tag; name "pmid19075198" defined multiple times with different content - ↑ Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U (2010). "Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study.". J Natl Cancer Inst 102 (2): 89-95. DOI:10.1093/jnci/djp436. PMID 20042716. Research Blogging.
- ↑ Van Gossum, Andre; Miguel Munoz Navas, Inaqui Fernandez-Urien, Cristina Carretero, Gerard Gay, Michel Delvaux, Marie Georges Lapalus, Thierry Ponchon, Horst Neuhaus, Michael Philipper, Guido Costamagna, Maria Elena Riccioni, Cristiano Spada, Lucio Petruzziello, Chris Fraser, Aymer Postgate, Aine Fitzpatrick, Friedrich Hagenmuller, Martin Keuchel, Nathalie Schoofs, Jacques Deviere (2009-07-16). "Capsule Endoscopy versus Colonoscopy for the Detection of Polyps and Cancer". N Engl J Med 361 (3): 264-270. DOI:10.1056/NEJMoa0806347. PMID 19605831. Retrieved on 2009-07-16. Research Blogging.
- ↑ (2007) "Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement". Ann. Intern. Med. 146 (5): 361-4. pmid=17339621. [e] PMID 17339621
- ↑ Campbell CL, Smyth S, Montalescot G, Steinhubl SR (2007). "Aspirin dose for the prevention of cardiovascular disease: a systematic review". JAMA 297 (18): 2018-24. DOI:10.1001/jama.297.18.2018. PMID 17488967. Research Blogging. PMID 17488967
- ↑ Flossmann E, Rothwell PM (2007). "Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies". Lancet 369 (9573): 1603-13. DOI:10.1016/S0140-6736(07)60747-8. PMID 17499602. Research Blogging. PMID 17499602
- ↑ Cole BF, Logan RF, Halabi S, et al (February 2009). "Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials". J. Natl. Cancer Inst. 101 (4): 256–66. DOI:10.1093/jnci/djn485. PMID 19211452. Research Blogging.
- ↑ Cooper K, Squires H, Carroll C, Papaioannou D, Booth A, Logan RF et al. (2010). "Chemoprevention of colorectal cancer: systematic review and economic evaluation.". Health Technol Assess 14 (32): 1-206. DOI:10.3310/hta14320. PMID 20594533. Research Blogging.
- ↑ Weingarten MA, Zalmanovici A, Yaphe J (2005). "Dietary calcium supplementation for preventing colorectal cancer and adenomatous polyps". Cochrane database of systematic reviews (Online) (3): CD003548. DOI:10.1002/14651858.CD003548.pub3. PMID 16034903. Research Blogging.
- ↑ Wactawski-Wende J, Kotchen JM, Anderson GL, et al (2006). "Calcium plus vitamin D supplementation and the risk of colorectal cancer". N. Engl. J. Med. 354 (7): 684-96. DOI:10.1056/NEJMoa055222. PMID 16481636. Research Blogging.
- ↑ Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP (2007). "Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial". Am. J. Clin. Nutr. 85 (6): 1586-91. PMID 17556697. [e]