Entecavir: Difference between revisions
imported>David E. Volk m (→External links) |
imported>David E. Volk mNo edit summary |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
[[Image:Entecavir structure.jpg| | |||
{{Chem infobox | |||
|align=right | |||
|image=[[Image:Entecavir structure.jpg|center|thumb|150px|{{#ifexist:Template:Entecavir structure.jpg/credit|{{Entecavir structure.jpg/credit}}<br/>|}}]] | |||
|width=150px | |||
|molname=entecavir | |||
|synonyms= Baraclude® | |||
|molformula= C<sub>12</sub>H<sub>15</sub>N<sub>5</sub>O<sub>3</sub> | |||
|molmass= 277.2792 | |||
|uses=Hepatitis B | |||
|properties=guanine analog, polymerase inhibitor | |||
|hazards=see drug interactions | |||
|iupac= see chemistry section | |||
|casnumber=142217-69-4 | |||
}} | |||
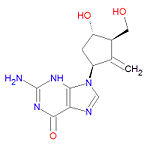
'''Entecavir''', sold under the brand name '''Baraclude®''' is an oral antiviral drug used to treat [[hepatitis B]] infection. Entecavir is a guanine analogue that inhibits all three steps in the viral replication process, and the manufacturer claims that it is more efficacious than previous agents used to treat hepatitis B ([[lamivudine]] and [[adefovir]]). In the triphosphate form, it competes with the natural DNA base [[deoxyguanosine triphosphate]] (dGTP) for incorporation into the negative and positive strand DNA synthesis. | '''Entecavir''', sold under the brand name '''Baraclude®''' is an oral antiviral drug used to treat [[hepatitis B]] infection. Entecavir is a guanine analogue that inhibits all three steps in the viral replication process, and the manufacturer claims that it is more efficacious than previous agents used to treat hepatitis B ([[lamivudine]] and [[adefovir]]). In the triphosphate form, it competes with the natural DNA base [[deoxyguanosine triphosphate]] (dGTP) for incorporation into the negative and positive strand DNA synthesis. | ||
Its IUPAC chemical name is 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-3H-purin-6-one and it has chemical formula C<sub>12</sub>H<sub>15</sub>N<sub>5</sub>O<sub>3</sub>. | == Chemistry == | ||
Its [[IUPAC]] chemical name is 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-3H-purin-6-one and it has chemical formula C<sub>12</sub>H<sub>15</sub>N<sub>5</sub>O<sub>3</sub>. | |||
==External links == | ==External links == | ||
{{CZMed}} | |||
Latest revision as of 20:38, 6 April 2009
|
| |||||||
| entecavir | |||||||
| |||||||
| Uses: | Hepatitis B | ||||||
| Properties: | guanine analog, polymerase inhibitor | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Entecavir, sold under the brand name Baraclude® is an oral antiviral drug used to treat hepatitis B infection. Entecavir is a guanine analogue that inhibits all three steps in the viral replication process, and the manufacturer claims that it is more efficacious than previous agents used to treat hepatitis B (lamivudine and adefovir). In the triphosphate form, it competes with the natural DNA base deoxyguanosine triphosphate (dGTP) for incorporation into the negative and positive strand DNA synthesis.
Chemistry
Its IUPAC chemical name is 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-3H-purin-6-one and it has chemical formula C12H15N5O3.
External links
The most up-to-date information about Entecavir and other drugs can be found at the following sites.
- Entecavir - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Entecavir - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Entecavir - Detailed information from DrugBank.