Gasoline/Citable Version
Gasoline or petrol is a fuel, derived from petroleum crude oil, for use in spark-ignited internal combustion engines. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms to those containing 11 or 12 carbon atoms. It has an initial boiling point at atmospheric pressure of about 35 °C (95 °F) and a final boiling point of about 200 °C (395 °F).[1][2][3][4] Gasoline is used primarily as fuel for the internal combustion engines in automotive vehicles as well in some small airplanes.
In Canada and the United States of America, the word "gasoline" is commonly used and it is often shortened to simply "gas" although it is a liquid rather than a gas. In fact, gasoline-dispensing facilities are referred to as "gas stations".
Most current or former Commonwealth countries use the term "petrol" and their dispensing facilities are referred to as "petrol stations". The term "petrogasoline" is also used sometimes. In some European countries and elsewhere, the term "benzin" (or a variant of that word) is used to denote gasoline.
In aviation, "mogas" (an abbreviation for "motor gasoline") is used to distinguish automotive vehicle fuel from aviation fuel known as "avgas".
Gasoline production from crude oil
Gasoline and other end-products are produced from petroleum crude oil in petroleum refineries. For a number of reasons it is very difficult to quantify the amount of gasoline produced by refining a given amount of crude oil:
- There are quite literally hundreds of different crude oil sources worldwide and each crude oil has its own unique mixture of thousands of hydrocarbons and other materials.
- There are also hundreds of crude oil refineries worldwide and each of them is designed to process a specific crude oil or a specific set of crude oils. Furthermore, each refinery has its own unique configuration of petroleum refining processes that produces its own unique set of gasoline blend components. Some crude oils have a higher proportion of hydrocarbons with very high boiling points than other crude oils and therefore require more complex refinery configurations to produce lower boiling point hydrocarbons that are usable in gasolines.
- There are a great many different gasoline specifications that have been mandated by various local, state or national governmental agencies.
- In many geographical areas, the amount of gasoline produced during the summer season (i.e., the season of the greatest demand for automotive gasoline) varies significantly from the amount produced during the winter season.
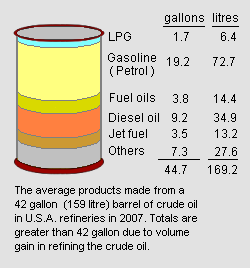
However, as an average of all the refineries operating in the United States in 2007,[5] refining a barrel of crude oil (i.e., 42 gallons or 159 litres) yielded 19.2 gallons (72.7 litres) of end-product gasoline as shown in the adjacent image. That is a volumetric yield of 45.7 percent. The average refinery yield of gasoline in other countries may be different.
From the viewpoint of performance when used in automotive spark-ignited internal combustion engines, the most important characteristic of a gasoline is its octane rating (discussed later in this article). Paraffinic hydrocarbons (alkanes) wherein all of the carbon atoms are in a straight chain have the lowest octane ratings. Hydrocarbons with more complicated configurations such as aromatics, olefins and branched paraffins have much higher octane ratings. To that end, many of the refining processes used in petroleum refineries are designed to produce hydrocarbons with those more complicated configurations.
Some of the most important refinery process streams that are blended together to obtain the end-product gasolines[6] are:
- Reformate (produced in a catalytic reformer): has a high content of aromatic hydrocarbons and a very low content of olefinic hydrocarbons (alkenes).
- Catalytically cracked gasoline (produced in a fluid catalytic cracker): has a high content of olefinic hydrocarbons and a moderate amount of aromatic hydrocarbons.
- Hydrocrackate (produced in a hydrocracker): has a moderate content of aromatic hydrocarbons.
- Alkylate (produced in an alkylation unit): has a high content of highly branched paraffinic hydrocarbons such as isooctane.
- Isomerate (produced in a catalytic isomerization unit): has a high content of the branched isomers of pentane and hexane.
Properties that determine the performance of gasoline
Octane rating
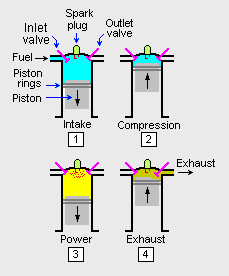
The adjacent image depicts what occurs in one of the combustion cylinders of a gasoline-fuelled, spark-ignited internal combustion engine operating in a 4-stroke cycle. Each cylinder in the engine has a movable piston that can slide upward and downward within the cylinder. Although not shown in the image, the bottom of the piston is connected to a rotating central crankshaft by a so-called connecting rod. The cycle starts with the piston at the top of the cylinder (i.e., where the piston is farthest away from the crankshaft axis) and the inlet and exhaust valves are closed. Then:
- During the intake stroke, the piston is pulled downward by the rotating crankshaft and the inlet valve opens to admit a mixture of fuel and air.
- During the compression stroke, the inlet valve closes and the piston is pushed upward by rotating crankshaft which compresses the fuel-air mixture.
- During the power stroke, the compressed fuel-air mixture is ignited by a spark from the spark plug. The resulting increase in temperature and pressure of the burning fuel forces the piston down which, in turn, forces the crankshaft to rotate.
- During the exhaust stroke, the outlet valve opens and the rotating crankshaft pushes the piston upward which forces the combustion product gases to be exhausted from the cylinder. That ends the 4-stroke cycle and the cycle then starts again.
In a typical multiple-cylinder engine, the timing of the each cylinder's cycle is such that the crankshaft is kept in continuous rotation.
If the gasoline spontaneously ignites and detonates (i.e., explodes) before it is ignited by the spark plug, it causes an abnormal phenomenon known as knocking, pinging or spark knock. The knocking is quite audible and prolonged knocking will damage an engine.
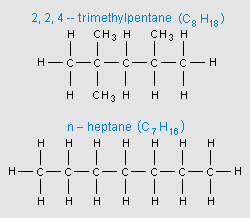
As briefly mentioned above, the most important performance characteristic of a gasoline is its octane rating, which is a measure of how resistant the gasoline is to knocking. In fact, the octane rating is sometimes referred to as the Anti-knock Index. The octane rating is based upon an arbitrary scale indexed relative to a liquid mixture of iso-octane (C8H18), which is 2,2,4-trimethylpentane, and n-heptane (C7H16). Iso-octane (see adjacent image), with a branched structure and a high resistance to knocking, has arbitrarily been assigned an octane rating of 100. N-heptane (see adjacent image), with a straight-chain structure and poor resistance to knocking has arbitrarily been assigned an octane rating of 0.
The octane rating of a specific gasoline is measured by using it in a single-cylinder test engine with a variable compression ratio and adjusting the ratio to produce a standard knock intensity as recorded by an instrument known as a knockmeter. By comparison to tabulated results from similar testing of various mixtures of iso-octane and n-heptane at the same compression ratio, the octane rating of the gasoline is determined. For example, if the gasoline test results match those of a mixture containing 90 volume % iso-octane and 10 volume % n-heptane, then the octane rating of the gasoline is taken to be 90.[7]
The octane rating is measured at two different operational conditions. The rating measured at the more severe operating conditions is called the Motor Octane Number (MON) [8] and the rating measured at the less severe conditions is called the Research Octane Number (RON) [9]. The Motor Octane Number is more representative of the performance of a gasoline when used in an automotive vehicle operated under load. For many gasoline formulations, the MON is about 8 to 10 points lower than the RON.
In the United States and Canada, the octane rating shown on the pumps in gasoline dispensing stations is the average of the gasoline's RON and the MON. That average is sometimes referred to as the Pump Octane Number (PON), the Anti-Knock Index (AKI), the Road Octane Number (RdON) and very often simply as (RON + MON)/2) or (R + M)/2. In Europe and Australia and other countries, the octane rating shown on the pumps is most often the RON.
As a broad generality, the higher is the compression ratio of a spark-ignited internal combustion engine, the higher is the performance level of the engine and the higher is the octane rating required for the gasoline fuel. The design of an engine determines its compression ratio and, therefore, the required gasoline octane. Using a gasoline with an octane rating higher than an engine requires will not improve the engine's performance, it will simply cost more.
Vapor pressure
The vapor pressure of a gasoline is a measure of its propensity to evaporate (i.e., its volatility) and high vapor pressures result in high evaporative emissions of smog-forming hydrocarbons which are undesirable from the environmental viewpoint. However, from the viewpoint of gasoline performance:
- The gasoline must be volatile enough that engines can start easily at the lowest expected temperature in the geographical area of the gasoline's expected market. For that reason, in most areas, gasoline marketed during the winter season has a higher vapor pressure than gasoline marketed in the summer season.
- Too high a volatility could cause excessive vapor leading to vapor locking in the fuel pump and fuel piping.
Thus, gasoline producers must provide gasolines that make possible the easy starting of engines and avoid vapor locking problems[10][11] while at the same time complying with the environmental regulatory limitations on hydrocarbon emissions.
Sulfur content
When gasoline is combusted, any sulfur compounds in the gasoline are converted into gaseous sulfur dioxide emissions which are undesirable from the environmental viewpoint. Some of the sulfur dioxide also combines with the water vapor formed when gasoline combusts and the result is the formation of an acidic, corrosive gas that can damage the engine and its exhaust system. Furthermore, sulfur interferes with the efficiency of the on-board catalytic converters (discussed later in this article).
Thus, sulfur compounds in gasoline are highly undesirable from either the environmental viewpoint or the engine performance viewpoint.[3][12][13] Many countries now mandate that the sulfur content of gasoline be limited to 10 ppm by weight.
Storage stability
Gasoline stored in fuel tanks and other containers will, in time, undergo oxidative degradation and form sticky resins referred to as gums. Such gums can precipitate out of the gasoline and cause fouling of the various components of internal combustion engines which reduces the performance of the engines and also makes it harder to start them. Relatively small amounts of various anti-oxidation additives are included in end-product gasoline to improve the gasoline stability during storage by inhibiting the formation of gums.
Other additives are also provided in end-product gasolines, such as corrosion inhibitors to protect gasoline storage tanks, freezing point depressants to prevent icing, and color dyes for safety or governmental regulatory requirements.[1][3][10]
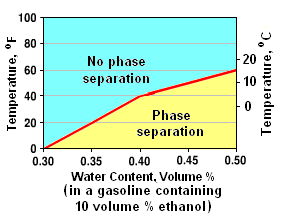
As discussed later in this article, many gasolines contain ethanol which is an alcohol with the formula C2H5OH. Gasoline is insoluble in water but ethanol and water are mutually soluble. Thus, end-product gasolines containing ethanol will, at certain temperatures and water concentrations, separate into a gasoline phase and an aqueous ethanol phase.[14]
For example, the adjacent graph shows that phase separation will occur in a gasoline, at temperatures of 5 to 16 °C (40 to 60 °F), containing 10 volume percent ethanol and as little as 0.40 to 0.50 volume percent water.
For the same temperature range, the fraction of water that an ethanol-containing gasoline can contain without phase separation increases with the percentage of ethanol. Thus, gasolines containing more than 10 volume percent ethanol will be less likely to experience phase separation.
Gasoline formulations and air quality regulations
In the United States
There is no "standard" composition or set of specifications for gasoline. In the United States, because of the complex national and individual state and local programs to improve air quality, as well as local refining and marketing decisions, petroleum refiners must supply fuels that meet many different standards. State and local air quality regulations involving gasoline overlap with national regulations and that leads to adjacent or nearby areas having significantly different gasoline specifications. According to a detailed study in 2006, [12] there were at least 18 different gasoline formulations required across the United States in 2002. Since many petroleum refiners in the United States produce three grades of fuel and the specifications for fuel marketed in the summer season vary significantly from the specifications in the winter season, that number may have been greatly understated. In any event, the number of fuel formulations has probably increased quite a bit since 2002. In the United States, the various fuel formulations are often referred to as "boutique fuels".[12][15][16] In general, most of the gasoline specifications meet the requirements of the so-called Reformulated Gasoline (RFG) mandated by federal law and implemented by the U.S. Environmental Protection Agency (U.S. EPA).
Some of the major properties and gasoline components focused upon by the various national and state or local regulatory programs are:
- Vapor pressure: The vapor pressure of a gasoline is of concern because evaporative emissions of the hydrocarbons in the gasoline lead to the formation of ozone in the atmosphere which reacts with vehicular and industrial emissions of gaseous nitrogen oxides (NOx) to form what is called photochemical smog. Smog is a combination of the words smoke and fog and traditionally referred to the mixture of smoke and sulfur dioxide that resulted from the burning of coal for heating buildings in places such as London, England during the 19th century and the first half of the 20th century. Modern photochemical smog does not come from coal burning but from vehicular and industrial emissions of hydrocarbons and nitrogen oxides. It appears as a brownish haze over large urban areas and is irritating to the eyes and lungs.
- Nitrogen oxides: Various nitrogen oxides (NOx) are formed during the combustion of gasoline in vehicles and the combustion of other fuels in industrial facilities. NOx is one of the ingredients involved in the atmospheric chemistry that produces photochemical smog and, as such, is a prominent air pollutant. In fact, it is one of the six so-called "criteria air pollutants" that are regulated by National Ambient Air Quality Standards (NAAQS) of the United States. The NOx emitted by vehicular engines using gasoline are largely controlled by the use of on-board devices, called catalytic converters, installed on most modern automobiles and other vehicles. They convert the NOx emissions into gaseous nitrogen and oxygen. They also convert any gaseous carbon monoxide emissions into gaseous carbon dioxide as well as converting any unburned gasoline hydrocarbons into gaseous carbon dioxide and water vapor.
- Toxic metals:
- Tetraethyl lead (TEL) — In the 1920's, petroleum refining technology was rather primitive and produced gasolines with an octane rating of about 40 – 60. But automotive engines were rapidly being improved and required better gasolines, which led to a search for octane rating enhancers. That search culminated in 1921[17][18][19] in the development of tetraethyl lead (TEL), a colorless, viscous liquid with the chemical formula of (CH3CH2)4Pb. Despite ethanol being widely recognized as an alternative anti-knock additive[19], the less expensive TEL quickly became commercially available as what was called TEL fluid, which contained 61.5 weight % TEL. The addition of as little as 0.8 ml of that TEL fluid per litre (equivalent to 0.5 gram of lead per litre) of gasoline resulted in significant octane rating increases. Production and sale of "leaded gas" was briefly banned in 1925 by the Surgeon General,[18][19] and a panel of experts was appointed to investigate a number of fatalities that had "occurred in the manufacture and mixing of the concentrated tetraethyl lead".[18] Then, in 1927, the Surgeon General set a voluntary standard for the petroleum refining industry to follow in mixing tetraethyl lead with gasoline. The standard was 3 cubic centimeters per gallon (cm3/gal), corresponding to the maximum then in use among refiners[18], and thus imposed no real restraint. For about the next 50 years, TEL was used as the most cost effective way to raise the octane rating of gasolines. During that period, petroleum refining technology grew until high-octane gasolines could, in fact, be produced without using TEL. Also, in about the 1940's, it was discovered that the lead being emitted in the exhaust gases from vehicular internal combustion engines was a toxic air pollutant that seriously affected human health. Because of its toxicity and the fact that catalytic converters being installed in vehicles could not tolerate the presence of lead, the U.S. EPA launched an initiative in 1972 to phase out the use of TEL in the United States and it was completely banned for use in on-road vehicles as of January 1996.[20][21] Using TEL in race cars, airplanes, marine engines and farm equipment is still permitted. TEL usage has also been phased out by most nations worldwide. As of 2008, the only nations still allowing extensive use of TEL are the Democratic People's Republic of Korea, Myanamar, and Yeman.[22][23]
- Methylcyclopentadienyl manganese tricarbonyl (MMT) — In Canada, MMT has been used as an octane enhancer in gasoline since 1976. It is also permitted for use as a gasoline octane enhancer in Argentina, Australia, Bulgaria, France, Russia, United States and conditionally in New Zealand. MMT is a yellow liquid with chemical formula of (CH3C5H4)Mn(CO)3. According to the U.S. EPA, ingested manganese is a required element of the diet at very low levels but it is also a neurotoxin and can cause irreversible neurological disease at high levels of inhalation.[24] The U.S. EPA has a concern that the use of MMT in gasoline could increase inhalation manganese exposures. After completing a 1994 risk evaluation on the use of MMT in gasoline, the U.S. EPA was unable to determine if there is a risk to the public health from exposure to emissions of MMT gasoline. As of now (2009), gasoline in the United States is allowed to contain MMT at a level equivalent to 0.00826 g/L (1/32 g/gallon) of manganese.[24] However, there are still many concerns about the possible adverse health effects from the usage of MMT and less than one percent of the gasoline marketed in the United States contains MMT.[25]
- Other toxic compounds: Gasoline contains some benzene (C6H6) which is an aromatic compound that is a known human carcinogen. For that reason, the amount of benzene in gasoline is limited by environmental regulations. In general, the combustion of aromatics can lead to the formation of other compounds that have deleterious effects on human health, such as aldehydes, butadiene, and polycyclic aromatic hydrocarbons (PAHs). Therefore, the total amount of aromatics in gasoline is also limited by environmental regulations.
- Olefins: Photochemical smog is formed by various atmospheric chemistry reactions between nitrogen oxides and what are called reactive hydrocarbons in the presence of sunlight. In the context of photochemical smog formation, some hydrocarbons are more reactive than others. For example, olefins are very reactive and methane is not reactive to any extent. For that reason, the olefin content of gasolines is limited by environmental regulations.
- Sulfur: Any sulfur compounds in gasoline will result in combustion exhaust emissions of sulfur dioxide to the atmosphere. Such emissions contribute to the formation of so-called acid rain and they also interfere with the on-board catalytic converters and reduce their efficiency. Therefore, the sulfur content of gasoline is limited by environmental regulations.
- Oxygen: Oxygen-containing compounds called oxygenates such methyl tert-butyl ether (MTBE) with a chemical formula of C5H12O or ethanol with a chemical formula of C2H5OH are added to gasolines for two reasons. The first reason is that the oxygen reduces the emissions of unburned hydrocarbons as well as the emissions of carbon monoxide. The second reason is that they significantly enhance the octane rating of gasolines which makes up for the octane rating loss resulting from the limiting of the high-octane aromatics and olefins as well as the banning of TEL usage.[2] MTBE was widely used during the 1990s as an oxygenate in the United States until it was found to be polluting underground water supplies. In the United States, it has now been largely replaced as an oxygenate by ethanol. Gasolines containing ethanol are now sold in every state in the United States and nearly half of the gasoline sold in the United States now contains up to 10 volume % ethanol either as an octane enhancer or to meet air quality requirements.[26]
As mentioned earlier above, there are a great many different sets of specifications or standards for gasolines marketed in the United States. The specifications tabulated below are those that have been mandated by law in the state of California (U.S. state). They are known as the California Reformulated Gasoline (CaRFG) Phase 3 Standards and are perhaps the most environmentally restrictive specifications in the United States:
| Property | Measurement unit |
Flat Limit (a) | Average Limit (a) |
|---|---|---|---|
| Reid vapor pressure (b) | psi (c) | 7.00 or 6.90 (d) | not applicable |
| Sulfur concentration | ppmw (e) | 20 | 15 |
| Benzene concentration | ppmv (e) | 0.8 | 0.7 |
| Aromatics concentration | ppmv | 25.0 | 22.0 |
| Olefins concentration | ppmv | 6.0 | 4.0 |
| Temperature at 50 volume % distilled (T50) | °F (f) | 213 | 203 |
| Temperature at 90 volume % distilled (T90) | °F | 305 | 295 |
| Oxygen concentration | weight % (g) | 1.8 – 2.2 | not applicable |
| Oxygenates other than ethanol | -- | prohibited | not applicable |
| (a)"Flat" limits apply to every batch of finished gasoline. "Average" limits allow specific batches to exceed the "flat" limits as long as the gasoline produced over a 180-day period meets the "average" limits and never exceeds the specified "cap" limits. (b) Reid vapor pressure (RVP) is measured as per ASTM method D-323 and differs slightly from the true | |||
Blendstock for Oxygenate Blending (BOB)
Some water usually exists in today's gasoline pipeline systems and in many gasoline storage facilities. Ethanol is very soluble in water and the resulting aqueous solutions of ethanol are very corrosive. For that reason, ethanol is not blended into gasoline at the producing petroleum refineries. Instead, ethanol is blended into gasoline at terminals near the end user markets.[30][31]
In other words, to meet the current specification required of reformulated gasolines, petroleum refiners in the United States basically produce blending stocks to which ethanol is added at terminals or other points at or near the end-user markets. A blendstock to be used in producing a reformulated gasolines is known as a BOB (Blendstock for Oxygenated Blending). A BOB to be used in producing a reformulated gasoline meeting the specifications mandated by the U.S. EPA is known as an RBOB. A BOB to be used in producing reformulated gasolines meeting the California specifications is known as a CaRBOB or CARBOB.[30][31]
In Canada
As of mid-2008, gasoline quality regulation in Canada is generally under provincial jurisdiction, except for some national jurisdiction over sulfur, benzene, lead, and the ability to require certain amounts of renewable fuels such as ethanol. Few provinces regulate many aspects of gasoline quality other than Reid vapor pressure. The exception to this is the province of Manitoba, which requires gasoline to comply with the voluntary national standard CGSB 3.5, Automotive Gasoline developed by the Canadian General Standards Board (CGSB), a component of Canada’s Department of Public Works and Government Services.[32]
The three gasoline quality limits mandated nationally are:
- Sulfur: 30 ppmw maximum
- Benzene: 1 volume % maximum
- Lead: Banned completely
The major details of the voluntary national standard CGSB 3.5, Automotive Gasoline are available in Appendix B of a report published in 2008.[32]
In Europe
The current standards developed by the European Union and the standards developed by the European Automobile Manufacturers Association (ACEA) are presented below. The individual countries in the European Union as well as any other European countries may have their own standards as well.
| Property | Measurement unit |
European Union Norm EN 228[33] |
ACEA Worldwide Fuel Charter[34] Category 4 gasoline |
|---|---|---|---|
| Octane Rating (a) range | -- | 90 | 87 – 93 |
| Vapor pressure | kPa | 45 – 90 (b) | 45 – 60 (f) |
| Sulfur concentration | mg/kg (c) | 10 | 10 |
| Benzene concentration | volume % | 1.0 | 1.0 |
| Aromatics concentration | volume % | 35.0 | 35.0 |
| Olefins concentration | volume % | 18.0 | 10.0 |
| Temperature at 10 volume % distilled (T10) | °C (d) | -- | 65 (f) |
| Temperature at 50 volume % distilled (T50) | °C | -- | 77 – 100 (f) |
| Temperature at 90 volume % distilled (T90) | °C | -- | 130 – 175 (f) |
| % evaporated at 70 °C (E70 summer) | volume % | 20 – 48 | -- |
| % evaporated at 70 °C (E70 winter) | volume % | 22 – 50 | -- |
| % evaporated at 70 °C (E70 ) | volume % | -- | 20 – 45 (f) |
| % evaporated at 100 °C (E100) | volume % | 46 – 71 | 50 – 65 (f) |
| % evaporated at 150 °C (E150) | volume % | 75 | -- |
| % evaporated at 180 °C (E180 ) | volume % | -- | 90 (f) |
| Final boiling point (FBP) | °C | 210 | 195 |
| Oxygen concentration | weight % | 2.7 (e) | 2.7 (g) |
| (a) Values are (Research Octane Number + Motor Octane Number) / 2 ... or simply (RON + MON) / 2 (b) Range is from summer minimum (45 kPa) to winter maximum (90 kPa) | |||
In Australia and New Zealand
The current gasoline standards developed by the national governments of Australia and New Zealand are presented below. The individual states in Australia may have also developed gasoline standards, and the same may be true of the regional councils in New Zealand.
| Property | Measurement unit |
Australia National Standard[35] |
New Zealand National Standard[36] |
|---|---|---|---|
| Octane Rating (a) | -- | 88 | 90 |
| Vapor pressure | kPa | -- (b) | 45 – 95 (c) |
| Flexible Volatility Index (d) | -- | -- (b) | 115 maximum |
| Sulfur concentration | mg/kg (e) | 50 | 150 (f) |
| Benzene concentration | volume % | 1.0 | 1.0 |
| Aromatics concentration | volume % | 42.0 | 42.0 |
| Olefins concentration | volume % | 18.0 | 18.0 |
| % evaporated at 70 °C (E70) | volume % | -- | 22 – 48 |
| % evaporated at 100 °C (E100) | volume % | -- | 45 – 70 |
| % evaporated at 150 °C (E150) | volume % | -- | 75 |
| % evaporated at 180 °C (E180 ) | volume % | -- | -- |
| Final boiling point (FBP) | °C | 210 | 210 |
| Oxygen concentration | weight % | 3.9 | -- |
| Ethanol | volume % | 10 (g) | 10 (h) |
| (a) Values are (Research Octane Number + Motor Octane Number) / 2 ... or simply (RON + MON) / 2 (b) Australian standard has no vapor pressure or volatility specification and no distillation specification | |||
In India
The gasoline quality standards below apply only to the major cities and there are plans for lowering the maximum sulfur content from 150 ppmw to 50 ppmw in the near future. The standards for the rural areas of India are significantly less stringent.
| Property | Measurement unit |
Regular Limit | Premium Limit |
|---|---|---|---|
| Octane Rating (b) range | -- | 86 | 90 |
| Vapor Lock Index (c) | -- | 750 – 950 | 750 – 950 |
| Sulfur concentration | ppmw | 150 | 150 |
| Benzene concentration | volume % | 1.0 | 1.0 |
| Aromatics concentration | volume % | 42.0 | 42.0 |
| Olefins concentration | volume % | 21.0 | 18.0 |
| Methyl tertiary-butyl ether (MTBE) | volume % | 15 | 15 |
| Temperature at 90 volume % distilled (T90) | °C | 150 | 150 |
| Temperature at 100 volume % distilled (FBP) | °C | 210 | 210 |
| Oxygen concentration | weight % | 2.7 | 2.7 |
| (a) Other less stringent standards are used for gasoline marketed in rural areas.
(b) Values are (Research Octane Number + Motor Octane Number) / 2 ... or simply (RON + MON) / 2 | |||
References
- ↑ 1.0 1.1 Gasoline FAQ - Part2 of 4, Bruce Hamilton, Industrial Research Ltd. (IRL), a Crown Research Institute of New Zealand.
- ↑ 2.0 2.1 Gary, J.H. and Handwerk, G.E. (2001). Petroleum Refining Technology and Economics, 4th Edition. Marcel Dekker, Inc.. ISBN 0-8247-0482-7.
- ↑ 3.0 3.1 3.2 The Relation Between Gasoline Quality, Octane Number and the Environment, Rafat Assi, National Project Manager of Jordan’s Second National Communications on Climate Change, presented at Jordan National Workshop on Lead Phase-out, United Nations Environment Programme, July 2008, Amman, Jordan.
- ↑ James Speight (2008). Synthetic Fuels Handbook, 1st Edition. McGraw-Hill, pages 92-93. ISBN 0-07-149023-X.
- ↑ Where Does My Gasoline Come from?, U.S. Department of Energy, Energy Information Administration, April 2008.
- ↑ See the schematic process flow diagram in the Petroleum refining processes article.
- ↑ Frank Kreith and D. Yogi Goswami (Editors) (2004). CRC Handbook of Mechanical Engineering, 2nd Edition. CRC Press. ISBN 0-8493-0866-6.
- ↑ As per the ASTM test method D2700
- ↑ As per the ASTM test method D2699
- ↑ 10.0 10.1 David S.J. Jones and Peter P. Pujado (Editors) (2006). Handbook of Petroleum Processing, First Edition. Springer. ISBN 1-4020-2819-9.
- ↑ John McKetta (Editor) (1992). Petroleum Processing Handbook. CRC Press. ISBN 0-8247-8681-5.
- ↑ 12.0 12.1 12.2 CRS Report for Congress "Boutique Fuels" and Reformulated Gasoline: Harmonization of Fuel Standards (May 10, 2006) , Brent D. Yacobucci, Congressional Research Service, Library of Congress
- ↑ Petrol and Diesel, Questions and Answers From website of New Zealand Ministry of Economic Development.
- ↑ E10 & E85 and Other Alternate Fuels Bruce Bauman, American Petroleum Institute (API)
- ↑ Boutique Fuels: State and Local Clean Fuels Programs From the website of the U.S. Environmental Protection Agency
- ↑ EPAct Section 1541 Boutique Fuels Report to Congress Report No. EPA420-R-06-901, December 2006, co-authored by the U.S. EPA and the U.S. Department of Energy.
- ↑ Definition of Tetraethyl Lead
- ↑ 18.0 18.1 18.2 18.3 Lead Poisoning: A Historical Perspective
- ↑ 19.0 19.1 19.2 Ethyl-leaded gasoline
- ↑ Phasing Lead Out of Gasoline a report issued by the United Nations Environmental Programme (UNEP)
- ↑ Prohibition on Gasoline Containing Lead or Lead Additives for Highway Use From the website of the U.S. Environmental Protection Agency
- ↑ Asia-Pacific Lead Matrix a report issued by the United Nations Environmental Programme (UNEP)
- ↑ West Asia, Middle East and North Africa Lead Matrix a report issued by the United Nations Environmental Programme (UNEP)
- ↑ 24.0 24.1 Comments on the Gasoline Additive MMT Retrieved from the U.S. EPA website on April 10, 2009
- ↑ Methylcyclopentadienyl Manganese Tricarbonyl (MMT): A Science and Policy Review Published by the International Council on Clean Transportation, January 2009
- ↑ E10 and other Low-Level Ethanol Blends From website of the U.S. Department of Energy.
- ↑ Final Regulation Order 2007 Amendments to California Phase 3 Reformulated Gasoline Regulation, California Code of Regulations, Title 13, Section 2260
- ↑ California Phase 3 Reformulated Gasoline (CaRFG) From the website of the California Air Resources Board.
- ↑ Miscellaneous Cleanup Amendments to the California Reformulated Gasoline Regulations From the website of the California Air Resources Board.
- ↑ 30.0 30.1 Appendix C: Using Ethanol in Gasoline Part of a report by the Energy Information Administration entitled Analysis of Selected Transportation Fuel Issues Associated with Proposed Energy Legislation - Summary
- ↑ 31.0 31.1 Methodology and Specifications Guide, 2008 A Platts publication.
- ↑ 32.0 32.1 Fuel Quality in Canada A 2008 report developed by The Pembina Institute for the Association of International Automobile Manufacturers of Canada
- ↑ European Norm 228 unleaded gasoline As of January 2009.
- ↑ Worldwide Fuel Charter September 2006, Category 4 gasoline, European Automobile Manufacturers Association (ACEA)
- ↑ Petrol Fuel Quality Standard As of October 2008. From website of Australian Government Department of the Environment, Water, Heritage and the Arts
- ↑ Requirements for Premium Grade Petrol Effective January 2006. From website of New Zealand Ministry of Economic Development.
- ↑ India Product Specifications - Gasoline Published by Asia Pacific Energy Consulting (APEC), June 2007
- Pages using ISBN magic links
- Subpages
- Engineering Extra Subpages
- Chemistry Extra Subpages
- Chemical Engineering Subgroup Citable Versions
- Environmental Engineering Subgroup Citable Versions
- Energy policy Subgroup Citable Versions
- Engineering Approved Extra Subpages
- Chemistry Approved Extra Subpages
- Citable versions of articles
- Engineering Citable Version Subpages
- Chemistry Citable Version Subpages
- All Content
- Engineering Content
- Chemistry Content
- Chemical Engineering tag
- Environmental Engineering tag
- Energy policy tag