User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok |
imported>Milton Beychok |
||
| Line 66: | Line 66: | ||
==History== | ==History== | ||
The phrase ''catalyzed processes'' was coined by [[Jöns Jakob Berzelius]] in 1836<ref> | The phrase ''catalyzed processes'' was coined by [[Jöns Jakob Berzelius]] in 1836<ref>{{cite book|author=Keith J. Laidler and John H. Meiser|Physical Chemistry|edition=1st Edition|publisher=Benjamin-Cummings Publishing|year=1982| pages=p.423|id=ISBN 0-8053-5682-7</ref> to describe reactions that are accelerated by substances that remain unchanged after the reaction. Other early chemists involved in catalysis were [[Alexander Mitscherlich]] who referred to ''contact processes'' and [[Johann Wolfgang Döbereiner]] who spoke of ''contact action'' and whose [[Lighter (fire starter)|lighter]] based on [[hydrogen]] and a [[platinum]] sponge became a huge commercial success in the 1820s. [[Humphry Davy]] discovered the use of platinum in catalysis. In the 1880s, [[Wilhelm Ostwald]] at [[Leipzig University]] started a systematic investigation into reactions that were catalyzed by the presence of [[acid]]s and [[base]]s, and found that chemical reactions occur at finite rates and that these rates can be used to determine the strengths of acids and bases. For this work, Ostwald was awarded the 1909 [[Nobel Prize in Chemistry]].<ref>{{cite journal|author=M.W. Roberts|title=Birth of the catalytic concept (1800-1900)|journal=Catalysis Letters| volume=67|issue=1|year=2000|pages=1–4|url =http://www.springerlink.com/content/qm3732u7x7577224/fulltext.pdf}}</ref> | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 11:12, 8 April 2010
In chemistry, catalysis is a process that uses a substance to accelerate the rate of a chemical reaction through an uninterrupted and repeated cycle of elementary steps until the last step regenerates the catalyst in its original form. The substance that does this is known as a catalyst. It is usually present in relatively small amounts and none of it is consumed in the process.[1]
Many substances can act as catalysts, including: metals, chemical compounds (e.g., metal oxides, sulfides, nitrides), organometallic complexes, and enzymes. Although a catalyst may be a gas, liquid or solid, most catalysts used in industrial chemical reactions are in the form of porous pellets. Since not all parts of a solid catalyst participate in the catalysis cycle, those parts that do participate are referred to as active sites. A single porous pellet may have 1018 active catalytic sites.[1]
This article does not discuss enzymatic or biochemical catalysis (for information on those types of catalysis, see the enzyme, biochemistry and organocatalysis articles).
The catalysis mechanism
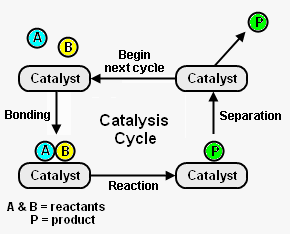
Figure 1 depicts the steps in a typical catalysis cycle. As depicted, the reactant molecules A and B are reacted to yield product P. The catalysis cycle starts with the bonding of reactant molecules A and B to the catalyst. A and B then react, while bound to the catalyst, to yield product P which is also bound to the catalyst. In the last step, the catalyst is regenerated by product P separating from the catalyst. The regenerated catalyst then begins cycle again by bonding with two more reactant molecules. [2]
In chemistry, activation energy[4] is the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a designated chemical reaction. It is denoted by Ea in units of kilojoules per mole (kJ/mol). It may be thought of as the energy barrier that must be overcome to start a chemical reaction.
For a chemical reaction to proceed at a reasonable rate, there should exist an appreciable number of reactant species (molecules, atoms, ions, etc.) with energy equal to or greater than the activation energy of the reaction.[3] A catalyst does not lower the activation energy for a reaction, instead it provides an alternative path for the reaction that has a lower activation energy. The catalyst changes the chemical kinetics of a reaction but not the chemical thermodynamics.
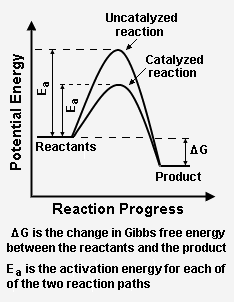
Figure 2 depicts how a catalyzed reaction follows a lower activation energy path than the higher activation energy path followed by the same reaction when it is not catalyzed. Overall, both the catalyzed path and the uncatalyzed path have the same change in Gibbs free energy between the reactants and the reaction product.
The energy diagram in Figure 2 illustrates several important points:[2]
- The presence of the catalyst provides an alternative reaction path, which is definitely more complex (see Figure 1), but energetically much more favorable.
- The activation energy of the catalyzed reaction is significantly smaller than that of the uncatalyzed reaction. Hence, the rate of the catalyzed reaction is much faster.
- Since the overall change in Gibbs free energy is the same for the catalyzed reaction as for the uncatalyzed reaction, the reaction equilibrium constant is not affected by the catalyst. As noted above, the catalyst does not change the chemical thermodynamics of the reaction. Thus, if a reaction is thermodynamically unfavorable, the catalyst cannot change that situation.
The term "turn over frequency" (TOF) is used quite commonly in the technical literature to characterize the activity of catalysts. However, the definition of TOF in the literature is not consistent and varies quite widely. For example, two of the definitions in the literature are:
- Moles of product formed per second per mole of catalyst[5]
- Moles of reactants converted per second per active site.[2]
In both of the above definitions, the unit of time is sometimes designated as an hour rather than a second.
Another catalytic activity term is the katal, an SI derived unit, which is used mostly in biochemistry to characterize the activity of enzymes.[6]
Types of catalysis
Catalysis can be categorized into two main types: heterogeneous and homogeneous. In heterogeneous catalysis, the catalyst is in one phase[7] while the reactants and products are in a different phase or, for some cases, two different phases. In homogeneous catalysis, the catalyst is in the same phase as the reactants and the products.[8]
Heterogeneous catalysis [8][9][10]
Typical examples of heterogeneous catalysis involve a solid catalyst with the reactants as either liquids or gases. Separation of the products from the catalyst is relatively easy. However, the catalyst in heterogeneous catalysis is often less selective than in homogeneous catalysis.
A few specific examples of heterogeneous catalysis are:[8][9][10]
- The catalytic converters in automobiles convert exhaust gases such as carbon monoxide (CO) and nitrogen oxides (NOx) into more harmless gases like carbon dioxide (CO2) and nitrogen (N2). Metals (solids) like platinum (Pt), palladium (Pd) and rhodium (Rh) are used as the catalyst. The metals are deposited as thin layers onto a ceramic honeycomb. This maximizes the surface area and keeps the amount of metal used to a minimum.
- The large-scale industrial processes for manufacturing sulfuric acid (H2SO4) involve using solid vanadium pentoxide (V2O5) as the catalyst to convert gaseous sulfur dioxide (SO2) into gaseous sulfur trioxide (SO3).
- The catalytic hydrogenation of liquid unsaturated hydrocarbons (alkenes) by reacting them with gaseous hydrogen (H2) to produce liquid saturated hydrocarbons (alkanes) uses metals like platinum (Pt) and palladium (Pd) as the catalyst. This is an example of three-phase catalysis, the catalyst being a solid and one of the reactants being a gas while another reactant and the product are liquids.
Homogeneous catalysis [8][9][10]
Typical examples of homogeneous catalysis have the catalyst, reactant and products all present as a gas or contained in a single liquid phase. Separation of the products from the catalyst is relatively difficult. However, the catalyst in homogeneous catalysis is often more selective than in heterogeneous catalysis.
A few examples of homogeneous catalysts are:[8][9][10]
- The depletion of ozone (O3) in the ozone layer of the Earth's atmosphere by chlorine free radicals (Cl·) is a an example where everything is present in a gas phase. The chlorine free radicals, derived from the slow breakdown of man-made chlorofluorohydrocarbons (CFCs) like CCl2F2 released into the atmosphere, acts both as a reactant and the catalyst in converting gaseous ozone to gaseous oxygen (O2).
- The production of an ester by reacting a carboxylic acid with an alcohol involves the use of sulfuric acid as the catalyst and is an example where everything is contained in a liquid phase. This reaction is known a Fischer esterification, named after the German chemist Hermann Emil Fischer (1852 - 1919).
Inhibitors, promoters and poisons
Applications
History
The phrase catalyzed processes was coined by Jöns Jakob Berzelius in 1836[11] to describe reactions that are accelerated by substances that remain unchanged after the reaction. Other early chemists involved in catalysis were Alexander Mitscherlich who referred to contact processes and Johann Wolfgang Döbereiner who spoke of contact action and whose lighter based on hydrogen and a platinum sponge became a huge commercial success in the 1820s. Humphry Davy discovered the use of platinum in catalysis. In the 1880s, Wilhelm Ostwald at Leipzig University started a systematic investigation into reactions that were catalyzed by the presence of acids and bases, and found that chemical reactions occur at finite rates and that these rates can be used to determine the strengths of acids and bases. For this work, Ostwald was awarded the 1909 Nobel Prize in Chemistry.[12]
References
- ↑ 1.0 1.1 Commission on Physical Sciences, Mathematics, and Applications (CPSMA), National Academies (1992). Catalysis Looks to the Future. National Academies Press. ISBN 0-309-04584-3. Available online at Executive Summary
- ↑ 2.0 2.1 2.2 I. Chorkendorff and J. W. Niemantsverdriet (2007). Concepts of Modern Catalysts and Kinetics, 2nd Edition. Wiley-VCH. ISBN 3-527-31672-8.
- ↑ 3.0 3.1 The Effect of Catalysts on Reaction Rates From a website provided by Jim Clarke, retired Head of Chemistry and then Head of Science at Truro School in Cornwall, United Kingdom.
- ↑ A term introduced in 1889 by the Swedish scientist Svante Arrhenius
- ↑ Hideo Kurosawa and Akio Yamamoto (2003). Fundamentals of Molecular Catalysis, 1st Edition. Elsevier Science. ISBN 0-444-50921-6.
- ↑ R. Dybkaer (2001). "Unit "katal" for Catalytic Activity (IUPAC Technical Report)". Pure Appl. Chem. 73 (6): 927-931.
- ↑ If a boundary exists between the catalyst and the reaction system (i.e., the reactants and the products), then the system has two phases. In this context, a phase is different from the most familiar states of matter: solid, liquids and gases. For example, if a liquid catalyst and a liquid reaction system were mutually insoluble, a boundary would exist between the catalyst and the reaction system. That would constitute two phases in the context of catalysis whereas it would be considered as being one state of matter, namely a liquid.
- ↑ 8.0 8.1 8.2 8.3 8.4 Types of Catalysis From a website provided by Jim Clarke, retired Head of Chemistry and then Head of Science at Truro School in Cornwall, United Kingdom.
- ↑ 9.0 9.1 9.2 9.3 Chemistry Explained Chemistry Encyclopedia
- ↑ 10.0 10.1 10.2 10.3 Catalyst Lecture 12 Faculty of Chemistry, Silesian University of Technology, Poland
- ↑ {{cite book|author=Keith J. Laidler and John H. Meiser|Physical Chemistry|edition=1st Edition|publisher=Benjamin-Cummings Publishing|year=1982| pages=p.423|id=ISBN 0-8053-5682-7
- ↑ M.W. Roberts (2000). "Birth of the catalytic concept (1800-1900)". Catalysis Letters 67 (1): 1–4.