Yersinia pestis: Difference between revisions
imported>Ariana Aronis |
mNo edit summary |
||
| (110 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{ | {{Taxobox | ||
| color = pink | |||
| name = ''Yersinia pestis'' | |||
| image = | |||
| regnum = Eubacteria | |||
| phylum = Proteobacteria | |||
| classis = Gamma Proteobacteria | |||
| ordo = Enterobacteriales | |||
| familia = Enterobacteriaceae | |||
| genus = ''Yersinia'' | |||
| species = ''Y. pestis'' | |||
| binomial = ''Yersinia pestis'' | |||
| binomial_authority = | |||
}} | |||
{{seealso|Plague}} | |||
''Yersinia pestis'' is a nonmotile, non–[[spore]]-forming, [[pleomorphic]], [[Gram-negative]], [[facultative anaerobic]], bipolar-staining bacillus [[bacterium]] belonging to the family ''[[Enterobacteriaceae]]''. It is also [[catalase]] positive and [[oxidase]] negative. The bacteria elaborate a [[lipopolysaccharide]] [[endotoxin]], [[coagulase]], and a [[fibrinolysin]], which are the principal factors in the [[pathogenesis]] of this [[disease]]. ''Yersinia pestis'' was discovered in 1894 by the Swiss/French physician and bacteriologist from the Pasteur Institute, [[Alexandre Yersin]]. It was isolated during an epidemic of the plague in Hong-Kong and It was Yersin who actually linked the plague with ''Yersinia pestis''. Originally named ''Pasteurella pestis'', the microbe was renamed in 1967 after its founder.<ref name=Iowa-YP>{{citation | |||
| url = http://www.uhl.uiowa.edu/services/ter/biological/agents/yersinia.xml | |||
| publisher=University of Iowa Hygienic Laboratory | |||
| title = Yersinia pestis (Plague) | year = 2008 | |||
| id = Former Reference 9}}</ref> | |||
== | ==Genome structure== | ||
The genetic material of ''Yersinia pestis'' is skein of circular DNA that is localized as the [[nucleoid]], which lacks a [[nuclear membrane]]. The [[genome]] structure has been decoded for two of the three sub-species of ''Yersinia pestis'', the KIM strain and the CO92 strain. The [[chromosome]] of the KIM strain contains 4,600,755 base pairs and the chromosome of the CO92 strain has 4,653,728 base pairs. There are 4,012 protein-coding genes, including 149 [[pseudogenes]]. The genome is rich in insertion sequences and displays anomalies in GC base-composition bias, which indicates frequent [[intragenomic recombination]]. <ref name=Parkhill>{{citation | |||
| url = http://www.ncbi.nlm.nih.gov/pubmed/11586360 | |||
| author = Parkhill J et al. | |||
| title = Genome sequence of ''Yersinia pestis'', the causative agent of plague | |||
| journal = Nature | |||
| date = 2001 Oct 4 | |||
| volume = 413 (6855) | |||
| pages = 523-7 | |||
| PMID = 11586360] | |||
| id = Former example 1}}</ref> | |||
Many genes seem to have been acquired from other bacteria and viruses, which suggests that ''Yersinia pestis'' is a pathogen that has undergone a large-scale genetic evolution. ''Yersinia pestis'' is also the host to the [[plasimds]] pCD1 (or pYV), pPCP1 (or pPst), and pMt1 (or pFra) which along with a [[pathogenicity island]] called HPI encode the [[proteins]] that cause the infamous pathogenicity of the bacteria. These virulence factors are essential for the invasion of the bacteria into the host, and the injection of its proteins into the cell. The pCD1 plasmid contains 70,300 bases with 4-8 copies per cell, pPCP1 has 9,600 bases with 100-200 copies per cell, and pMT1 has 96,200 bases with 1-2 copies per cell. The pMt1 plasmid encodes the F1 [[polysaccharide]] that forms the lumpy surface feature of the bacteria. This means that the chromosome is about 48 times the size of the biggest plasmid.<ref name=Parkhill /> | |||
==Cell structure and metabolism== | |||
''Yersinia pestis'' contains a [[cell wall]] that consists of an outer membrane that compromises an inner [[phospholipid layer]] and an outer [[lipopolysaccharide]] layer. There is also a middle [[peptidoglycan]] layer that lies exterior to the [[plasma membrane]]. The bacteria's cell wall is quite unlike the typical Gram-negative enterobacterial cell wall as it lacks O-side chains due to a disrupted O-antigen [[gene cluster]].<ref name=Prentice>{{citation | |||
| url = http://www.rkm.com.au/BACTERIA/Yersinia-pestis.html | |||
| author = Prentice, Mike | |||
| title = Yersinia pestis (plague, Black Death, Bubonic Plague) | |||
| id = Former example 6 | |||
}}</ref> | |||
Under some circumstances, ''Yersinis pestis'' may be surrounded by a loose [[capsule]] and is covered by a [[slime envelope]] that is heat labile. The [[cytoplasm]] of the bacteria is filled with [[ribosomes]] are linked into [[polysomes]]. There is also a hypothesis that [[type III secretion needles]] may be demonstrated in ''Yersinia pestis'' at 37 [[Celsius (unit)|°C]] (98.6 [[Fahrenheit (unit)|°F]]) and that their formation is triggered when it comes in contact with other cells. Such needles have been demonstrated in its closely related species, ''Yersinia enterocolitica''.<ref name=Prentice /> | |||
==Ecology== | |||
''Yersinia pestis'' is a [[Gram-negative bacteria]] that is a [[facultative anaerobe]]. During an outbreak the bacteria can survive for long periods of time in cool, moist areas such as the soil of rodent holes. It is easily destroyed by sunlight and drying, but even so when released into the air it may survive for up to one hour. The optimum temperature for growth of the bacteria is about 26 °C (78.8 °F) to 37 °C (98.6 °F). Between outbreaks the bacteria is believed to circulate within populations of several rodent species without causing excessive death. Such groups of infected animals serve as silent, long-term carriers of the infection.<ref name=Doepel>{{citation | |||
| url = http://www3.niaid.nih.gov/news/newsreleases/1996/plague.htm | |||
| author = Doepel, Laurie K. | |||
| publisher = National Institute of Allergy and Infectious Diseases (NIAID) | |||
| title = Scientists Identify Genes Critical to Transmission of Bubonic Plague | |||
| date = July 18, 1996 | |||
| id = former citation 4}}</ref> | |||
The plague caused by ''Yersinia pestis'' is a [[zoonotic]] disease primarily affecting rodents. Humans do not play a role in the long-term survival of the bacterium. Fleas are the [[vectors]] of transmission in rodents as well as from rodents to humans. The most common method of transmission of the bacteria to humans is through the bite of an infected flea. After the fleas feed with a blood meal, they are believed to regurgitate bacteria back into uninfected animals.<ref name=Chamberlain>{{citation | |||
| url = http://www.kcom.edu/faculty/chamberlain/website/lectures/lecture/plague.htm | |||
| author = Chamberlain, Neil R. | title = Plague | date = 8/3/2004. | |||
| id = Former reference 10 | |||
}}</ref> | |||
''Yersinia pestis'' contains [[enzootic]] and [[epizootic]] transmission cycles involving rodents and their fleas. Risk for plague in humans is greatest during the epizootic cycle because the mortality rate for rats is high, causing the fleas to seek alternative hosts, including humans. The plague caused by ''Yersinia pestis'' can be separated into the Urban and Sylvatic cycles. In the Urban cycle the flea hosts are compromised of domestic rodents, while in the Sylvatic cycle the hosts are wild rodent populations.<ref name=Chamberlain /> | |||
[[ | ==Pathology== | ||
''Yersinia pestis'' is transmitted to people that have been bitten by infected fleas that are carried on rodents, most commonly which are rats, field mice, squirrel prairie dogs, rabbits and even animals such as cats and camels. The most common vector is the is the rat flea ''[[Xenopsylla cheopis]]'', although ticks and human lice have been identified as possible vectors. Humans are accidental hosts in the natural cycle of this disease. | |||
=== | ''Y. pestis'' has no effect on the flea host. The reason for this is that in order for the bacteria to produce the two [[antiphagocytic]] components (F1 antigen and the VW antigens, both which are required for virulence) it needs to be in a temperature of at 37 °C and not lower. The flea has a body temperature around 25 °C.<ref name=Schoenstadt>{{citation | ||
| url = http://plague.emedtv.com/yersinia-pestis/yersinia-pestis-p2.html | |||
| author = Schoenstadt, Arthur | |||
| title = Yersinia Pestis | date = October 14, 2006 | |||
|id=former example 2}}</ref> | |||
[[Image:Xenopsylla_cheopsis.jpg]] | |||
When the flea ingests blood that is infected with ''Yersinia pestis'', it produces a [[lipopolysaccharide]] [[endotoxin]], [[coagulase]], which causes the blood to clot and the bacteria multiply to the thousands. While the bacteria grows in the flea it loses its capsule layer and while most of the organisms are phagocytosed, a few are taken up by tissue [[macrophages]], which are unable to kill the bacteria. The bacteria kill the macrophage and migrate to the [[lymph nodes]] where they are phagocytosed by the [[polymorphonuclear cells]] and [[mononuclear phagocytes]], and multiply intracellularly. Afterwards, with lysis the bacteria can invade distant organs and continue to multiply. The [[hem binding]] chromosomal [[locus]] is turned on which causes the aggregation of [[planar molecules]] thus creating a block in the stomach causing the flea to be blood-frenzy. During these subsequent blood feedings, the bacteria are inoculated in a host’s and once the infection has spread to the lungs, pneumonia is developed. Through sneezing and coughing the bacteria can now spread into the air and contaminate new hosts.<ref name=Minnaganti>{{citation | |||
| url = http://www.emedicine.com/med/topic3381.htm | |||
| author = Minnaganti, Venkat R. | |||
| title = Plague | |||
| date = May 12, 2006 | journal = eMedicine | |||
| id = former citation 5}}</ref> | |||
The bacteria is able to inhibit [[phagocytosis]], [[inflammation]], and induce [[apoptosis]] of macrophages. ''Yersinia pestis'' contains 29 different [[Ysc proteins]] which assemble to form a [[pore]] in the inner and outer membrane of the bacteria. Once the bacterium makes contact with a cell, certain translocator Yops form a pore and then go across the channel through the bacterial and eukaryotic membranes to obtain access to the cell's [[cytoplasm]]. There are at least 6 different effector Yops which when transported into the eukaryotic cells, prevent phagocytosis. These proteins also encode the [[V antigen]] that appears to have immunosuppressive effects on the host's immune system.<ref name=Chamberlain /> | |||
The symptoms that the infected individuals have vary according to the type of plague that person is inflicted with. The plagues are all caused by ''Yersinia pestis'' but differ in how the person was infected, how the disease is transmitted and which organs the bacteria resides in. | |||
==Biological warfare== | |||
{{seealso|Biological weapon}} | |||
''Yersinia pestis'' has long been considered a possible biological warfare agent, and was used, with a flea vector, in China by Japanese forces in the [[Second World War]]. It is in the [[Select Agent Program]] and [[CDC Bioterrorism Agents-Disease list]], considered a likely choice for a bioweapon, as it is very easy to spread and is resistant to multiple drugs. Both the USA and former USSR researched and developed methods of aerosolizing the plague. It was, however, never made into a deliverable weapon by the U.S. or U.K., and there are conflicting reports if the Soviets weaponized it. Reasons for this included difficulty in maintaining virulence, as well as the basic problem that aerosol delivery, other than with point warheads, can reach A reach unintended targets. Hence the USA program was terminated in 1969.<ref name=Bellazzini>{{citation | |||
|url = http://www.residentandstaff.com/issues/articles/2006-03_01.asp | |||
| author = Bellazzini, Marc A; Myint, Michael | |||
| title = ''Yersinia Pestis'': New Challenges in the Age of Bioterrorism” | |||
| date = March 2006 | |||
| volume = 56 | issue = 3 | journal = Resident and Staff | |||
| id = Former reference 7}}</ref> | |||
Because of the delay between exposure to the bacteria and signs of illness, people could travel over a large area before becoming contagious and possibly infect others. It is also possible to be employed as a bioweapon, because the bacterium occurs in nature and could very easily be isolated and grown in a laboratory. If used as an aerosol attack it could cause cases of the pneumonic form of the plague from one to six days after infection. However manufacturing such a weapon requires further advanced knowledge and technology. <ref name= FAQ>{{citation | |||
| url = http://www.bt.cdc.gov/agent/plague/faq.asp | |||
| author = Coordinating Center for Infectious Diseases (CCID). National Center for Zoonotic, Vector-Borne, and Enteric Diseases (NCZVED) Division of Vector-Borne Infectious Diseases | |||
| title = Frequently Asked Questions about Plague | date = April 5, 2005}}</ref> | |||
Use of the organism as a [[biological weapon]] began as early as the 14th century when the Tartar armor laid siege to Caffa, Crimea. When an outbreak ravaged the Tartars, they catapulted plague-ridden corpses over the walls and into the city so that the disease would spread to their enemies. Genoese defenders then fled to Italy which spread the disease that devastated Europe in the 14th century.<ref name=Smith>{{citation | |||
| url = http://www.loyno.edu/~history/journal/1996-7/Smith.html | |||
| author = Smith, Christine A. | |||
| title = Plague in the Ancient World: A Study from Thucydides to Justinian | year = 1996 | |||
| id = Former reference 11}}</ref> | |||
During 1930-1940 general [[Shiro Ishii]] of the Japanese imperial Army [[Unit 731]] spread disease out of proportion. Unable to use aerosol or water, he dropped ceramic bombs with plague infested fleas over populated areas of China causing outbreaks.<ref name=Bellazzini /> Ceramic bombs containing infected lice were used; there are mixed reports of their effectiveness. | |||
== | ==History== | ||
Traces of outbreaks of the plague go as far back as to ancient times and specifically 5th century BC [[Athens]] and [[Sparta]]. During the [[Peleponnesian War]] fought between those two city-states there was an outbreak that was recorded by the historian [[Thucydides]].<ref name=Smith /> | |||
In 541 AD the plague stuck the [[Byzantine Empire]], killing more than 100 million people during a 50-year period. A fourth of the Mediterranean's population was destroyed. Outbreaks of the plague continued to strike the Mediterranean each subsequent generation up until 750 AD.<ref name=Smith /> | |||
In the 14th century the plague returned and struck Europe, causing the most well known pandemic in history known as the "Black Death" killing one third of Europe's population and about 75 million people worldwide. As mentioned earlier, after Genoese trading ships fled from [[Caffa]], they reached the port of [[Messina]] in Italy. When the ship arrived all the crew members were either infected or dead and it is assumed that there were infected rats and fleas aboard as well. From the port the plague spread to Genoa and Venice by 1348. From Italy the disease spread to France, Spain, Portugal, and England and from 1348-1351 spread east to Germany, Scandinavia and Russia. The period of the [[black plague]] lasted from 1347-1351. After this period the plague returned each consecutive generation with varying virulence up until the 1700's. During that time period more than 100 plague epidemics spread across Europe.<ref name=Smith /> | |||
The third pandemic occurred in China in 1855 and killed more than 12 million people in China and India alone. This pandemic was considered active up until 1959 and is thought to have spread by trade routes and migration of populations to southern China.<ref name=Smith /> | |||
The last urban plague epidemic that included human-to-human transmission occurred in Los Angeles in 1924-25. Since then the USA has had cases of the plague in scattered rural areas, mostly in two regions: northern New Mexico, northern Arizona, and southern Colorado; and California, southern Oregon, and far western Nevada, and these were acquired from wild rodents and their fleas. Annually, about 10 to 15 cases of the plague are reported in the USA and globally the World Health Organization reports 1,000 to 3,000 cases.<ref name=Schoenstadt /> | |||
== | ==Treatment== | ||
The first treatment measure would be to take [[antibiotics]] within 24 hours of the first signs of symptoms. The most common oral medications used for curing and preventing the disease are [[tetracycline]], [[doxycycline]], and a [[fluoroquinolone]]. For injection or intravenous use, [[streptomycin]] or [[gentamicin]] antibiotics are used.<ref name=Bellazzini /> | |||
= | People who have had close contact with an infected person can reduce the chances of becoming ill by beginning treatment within 7 days of their exposure. Treatment consists of taking the mentioned antibiotics for at least 7 days.<ref name=Bellazzini /> | ||
== | A [[formalin inactivated]] [[vaccination]] is available for adults who had a higher chance of contracting the disease. However, its effectiveness is limited and it can cause severe inflammation. Scientists are currently experimenting with a recombinant [[fusion protein]] of the ''Yersinia pestis'' F1 and V [[antigens]]. Natural or induced immunity can be achieved by the production of specific [[opsonins|opsonic]] antibodies against F1 and V antigens. However bacteria that lack the F1 antigen are still virulent, and the V antigens are sufficiently variable, so that vaccines composed of these antigens may not be fully protective against all strains of the bacteria.<ref name=>{{citation | ||
| url = http://www.cdc.gov/mmwr/preview/mmwrhtml/00044836.htm | |||
| author = Gage, Kenneth L, Dennis, David T, Tsai, Theodore F. | |||
| title = Prevention of Plague: Recommendations of the Advisory Committee on Immunization Practices (ACIP) | |||
| publisher = Centers for Disease Control | date = 9/9/1998}}</ref> | |||
==Current Research== | ==Current Research== | ||
Plague research is being conducted by several government agencies in an effort to help in the diagnosis, treatment, and prevention caused by ''Yersinia pestis'', as well as addressing the need to defend against deliberately caused disease outbreaks. Specifically this research focuses on developing a [[vaccine]] against the pneumonic plague, developing [[antibiotic]]s to prevent and treat infection, and most importantly studying and identifying genes and proteins in ''Yersinia pestis'' that infect the digestive tract of fleas and enable them to grow and function in humans.<ref name=Doepel /> | |||
Specifically, scientists at the National Institute of Allergy and Infectious Diseases (NIAID) Rocky Mountain Laboratories (RML), found that three [[gene]]s in ''Yersinia pestis'' change it from a harmless, long-term inhabitant in the flea’s mid gut to one that migrates and accumulates in its foregut. As a result of this change, the flea begins to starve, causing it to fanatically feed, during which it regurgitates the bacteria and hence transmits the plague. Although it was known for quite some time that the bacterium’s transmission is dependant on the fleas as hosts, there was little understanding about the molecular and genetic mechanisms by which this [[colonization occurs]]. They began experiments on three [[hemin]] storage genes (''hms''), which are abundant in red blood cells, and acts as the iron-containing part of the [[hemoglobin]] molecule that binds oxygen. To understand the role of these genes in the host Dr. Hinnebusch conducted experiments with oriental rat fleas, in which he injected the normal ''Yersinia pestis'' bacteria and a mutant form which was missing the ''hms'' genes. After four weeks, the scientists found that only those fleas infected with the normal bacteria developed the foregut blockage, which was accompanied by a high rate of mortality. These results indicated that the ''hms'' genes are required for ''Yersinia pestis'' to cause the foregut blockage.<ref name=Doepel /> | |||
Next, the scientists highlighted both forms of ''Yersinia pestis'' with fluoresce green and after dissecting the host fleas, they noted how the mutant bacteria remained in the midgut while the normal bacteria had migrated to the foregut in many fleas which eventually, became packed with bacteria. Now other genes are being studied that may affect the bacteria’s ability to transmit infection and it was observed that the blockage that develops in the flea foregut breaks down at temperatures above 26.66 °C (80 °F) to 29.44 °C (85 °F). Scientists are trying to determine why this occurs and if such temperature changes might suppress the products of ''hms'' or other genes.<ref name=Doepel /> | |||
==References== | ==References== | ||
[ | {{reflist|2}} | ||
[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 17:01, 9 November 2024
| Yersinia pestis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Yersinia pestis |
- See also: Plague
Yersinia pestis is a nonmotile, non–spore-forming, pleomorphic, Gram-negative, facultative anaerobic, bipolar-staining bacillus bacterium belonging to the family Enterobacteriaceae. It is also catalase positive and oxidase negative. The bacteria elaborate a lipopolysaccharide endotoxin, coagulase, and a fibrinolysin, which are the principal factors in the pathogenesis of this disease. Yersinia pestis was discovered in 1894 by the Swiss/French physician and bacteriologist from the Pasteur Institute, Alexandre Yersin. It was isolated during an epidemic of the plague in Hong-Kong and It was Yersin who actually linked the plague with Yersinia pestis. Originally named Pasteurella pestis, the microbe was renamed in 1967 after its founder.[1]
Genome structure
The genetic material of Yersinia pestis is skein of circular DNA that is localized as the nucleoid, which lacks a nuclear membrane. The genome structure has been decoded for two of the three sub-species of Yersinia pestis, the KIM strain and the CO92 strain. The chromosome of the KIM strain contains 4,600,755 base pairs and the chromosome of the CO92 strain has 4,653,728 base pairs. There are 4,012 protein-coding genes, including 149 pseudogenes. The genome is rich in insertion sequences and displays anomalies in GC base-composition bias, which indicates frequent intragenomic recombination. [2]
Many genes seem to have been acquired from other bacteria and viruses, which suggests that Yersinia pestis is a pathogen that has undergone a large-scale genetic evolution. Yersinia pestis is also the host to the plasimds pCD1 (or pYV), pPCP1 (or pPst), and pMt1 (or pFra) which along with a pathogenicity island called HPI encode the proteins that cause the infamous pathogenicity of the bacteria. These virulence factors are essential for the invasion of the bacteria into the host, and the injection of its proteins into the cell. The pCD1 plasmid contains 70,300 bases with 4-8 copies per cell, pPCP1 has 9,600 bases with 100-200 copies per cell, and pMT1 has 96,200 bases with 1-2 copies per cell. The pMt1 plasmid encodes the F1 polysaccharide that forms the lumpy surface feature of the bacteria. This means that the chromosome is about 48 times the size of the biggest plasmid.[2]
Cell structure and metabolism
Yersinia pestis contains a cell wall that consists of an outer membrane that compromises an inner phospholipid layer and an outer lipopolysaccharide layer. There is also a middle peptidoglycan layer that lies exterior to the plasma membrane. The bacteria's cell wall is quite unlike the typical Gram-negative enterobacterial cell wall as it lacks O-side chains due to a disrupted O-antigen gene cluster.[3]
Under some circumstances, Yersinis pestis may be surrounded by a loose capsule and is covered by a slime envelope that is heat labile. The cytoplasm of the bacteria is filled with ribosomes are linked into polysomes. There is also a hypothesis that type III secretion needles may be demonstrated in Yersinia pestis at 37 °C (98.6 °F) and that their formation is triggered when it comes in contact with other cells. Such needles have been demonstrated in its closely related species, Yersinia enterocolitica.[3]
Ecology
Yersinia pestis is a Gram-negative bacteria that is a facultative anaerobe. During an outbreak the bacteria can survive for long periods of time in cool, moist areas such as the soil of rodent holes. It is easily destroyed by sunlight and drying, but even so when released into the air it may survive for up to one hour. The optimum temperature for growth of the bacteria is about 26 °C (78.8 °F) to 37 °C (98.6 °F). Between outbreaks the bacteria is believed to circulate within populations of several rodent species without causing excessive death. Such groups of infected animals serve as silent, long-term carriers of the infection.[4]
The plague caused by Yersinia pestis is a zoonotic disease primarily affecting rodents. Humans do not play a role in the long-term survival of the bacterium. Fleas are the vectors of transmission in rodents as well as from rodents to humans. The most common method of transmission of the bacteria to humans is through the bite of an infected flea. After the fleas feed with a blood meal, they are believed to regurgitate bacteria back into uninfected animals.[5]
Yersinia pestis contains enzootic and epizootic transmission cycles involving rodents and their fleas. Risk for plague in humans is greatest during the epizootic cycle because the mortality rate for rats is high, causing the fleas to seek alternative hosts, including humans. The plague caused by Yersinia pestis can be separated into the Urban and Sylvatic cycles. In the Urban cycle the flea hosts are compromised of domestic rodents, while in the Sylvatic cycle the hosts are wild rodent populations.[5]
Pathology
Yersinia pestis is transmitted to people that have been bitten by infected fleas that are carried on rodents, most commonly which are rats, field mice, squirrel prairie dogs, rabbits and even animals such as cats and camels. The most common vector is the is the rat flea Xenopsylla cheopis, although ticks and human lice have been identified as possible vectors. Humans are accidental hosts in the natural cycle of this disease.
Y. pestis has no effect on the flea host. The reason for this is that in order for the bacteria to produce the two antiphagocytic components (F1 antigen and the VW antigens, both which are required for virulence) it needs to be in a temperature of at 37 °C and not lower. The flea has a body temperature around 25 °C.[6]
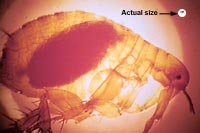
When the flea ingests blood that is infected with Yersinia pestis, it produces a lipopolysaccharide endotoxin, coagulase, which causes the blood to clot and the bacteria multiply to the thousands. While the bacteria grows in the flea it loses its capsule layer and while most of the organisms are phagocytosed, a few are taken up by tissue macrophages, which are unable to kill the bacteria. The bacteria kill the macrophage and migrate to the lymph nodes where they are phagocytosed by the polymorphonuclear cells and mononuclear phagocytes, and multiply intracellularly. Afterwards, with lysis the bacteria can invade distant organs and continue to multiply. The hem binding chromosomal locus is turned on which causes the aggregation of planar molecules thus creating a block in the stomach causing the flea to be blood-frenzy. During these subsequent blood feedings, the bacteria are inoculated in a host’s and once the infection has spread to the lungs, pneumonia is developed. Through sneezing and coughing the bacteria can now spread into the air and contaminate new hosts.[7]
The bacteria is able to inhibit phagocytosis, inflammation, and induce apoptosis of macrophages. Yersinia pestis contains 29 different Ysc proteins which assemble to form a pore in the inner and outer membrane of the bacteria. Once the bacterium makes contact with a cell, certain translocator Yops form a pore and then go across the channel through the bacterial and eukaryotic membranes to obtain access to the cell's cytoplasm. There are at least 6 different effector Yops which when transported into the eukaryotic cells, prevent phagocytosis. These proteins also encode the V antigen that appears to have immunosuppressive effects on the host's immune system.[5]
The symptoms that the infected individuals have vary according to the type of plague that person is inflicted with. The plagues are all caused by Yersinia pestis but differ in how the person was infected, how the disease is transmitted and which organs the bacteria resides in.
Biological warfare
- See also: Biological weapon
Yersinia pestis has long been considered a possible biological warfare agent, and was used, with a flea vector, in China by Japanese forces in the Second World War. It is in the Select Agent Program and CDC Bioterrorism Agents-Disease list, considered a likely choice for a bioweapon, as it is very easy to spread and is resistant to multiple drugs. Both the USA and former USSR researched and developed methods of aerosolizing the plague. It was, however, never made into a deliverable weapon by the U.S. or U.K., and there are conflicting reports if the Soviets weaponized it. Reasons for this included difficulty in maintaining virulence, as well as the basic problem that aerosol delivery, other than with point warheads, can reach A reach unintended targets. Hence the USA program was terminated in 1969.[8]
Because of the delay between exposure to the bacteria and signs of illness, people could travel over a large area before becoming contagious and possibly infect others. It is also possible to be employed as a bioweapon, because the bacterium occurs in nature and could very easily be isolated and grown in a laboratory. If used as an aerosol attack it could cause cases of the pneumonic form of the plague from one to six days after infection. However manufacturing such a weapon requires further advanced knowledge and technology. [9]
Use of the organism as a biological weapon began as early as the 14th century when the Tartar armor laid siege to Caffa, Crimea. When an outbreak ravaged the Tartars, they catapulted plague-ridden corpses over the walls and into the city so that the disease would spread to their enemies. Genoese defenders then fled to Italy which spread the disease that devastated Europe in the 14th century.[10]
During 1930-1940 general Shiro Ishii of the Japanese imperial Army Unit 731 spread disease out of proportion. Unable to use aerosol or water, he dropped ceramic bombs with plague infested fleas over populated areas of China causing outbreaks.[8] Ceramic bombs containing infected lice were used; there are mixed reports of their effectiveness.
History
Traces of outbreaks of the plague go as far back as to ancient times and specifically 5th century BC Athens and Sparta. During the Peleponnesian War fought between those two city-states there was an outbreak that was recorded by the historian Thucydides.[10]
In 541 AD the plague stuck the Byzantine Empire, killing more than 100 million people during a 50-year period. A fourth of the Mediterranean's population was destroyed. Outbreaks of the plague continued to strike the Mediterranean each subsequent generation up until 750 AD.[10]
In the 14th century the plague returned and struck Europe, causing the most well known pandemic in history known as the "Black Death" killing one third of Europe's population and about 75 million people worldwide. As mentioned earlier, after Genoese trading ships fled from Caffa, they reached the port of Messina in Italy. When the ship arrived all the crew members were either infected or dead and it is assumed that there were infected rats and fleas aboard as well. From the port the plague spread to Genoa and Venice by 1348. From Italy the disease spread to France, Spain, Portugal, and England and from 1348-1351 spread east to Germany, Scandinavia and Russia. The period of the black plague lasted from 1347-1351. After this period the plague returned each consecutive generation with varying virulence up until the 1700's. During that time period more than 100 plague epidemics spread across Europe.[10]
The third pandemic occurred in China in 1855 and killed more than 12 million people in China and India alone. This pandemic was considered active up until 1959 and is thought to have spread by trade routes and migration of populations to southern China.[10]
The last urban plague epidemic that included human-to-human transmission occurred in Los Angeles in 1924-25. Since then the USA has had cases of the plague in scattered rural areas, mostly in two regions: northern New Mexico, northern Arizona, and southern Colorado; and California, southern Oregon, and far western Nevada, and these were acquired from wild rodents and their fleas. Annually, about 10 to 15 cases of the plague are reported in the USA and globally the World Health Organization reports 1,000 to 3,000 cases.[6]
Treatment
The first treatment measure would be to take antibiotics within 24 hours of the first signs of symptoms. The most common oral medications used for curing and preventing the disease are tetracycline, doxycycline, and a fluoroquinolone. For injection or intravenous use, streptomycin or gentamicin antibiotics are used.[8]
People who have had close contact with an infected person can reduce the chances of becoming ill by beginning treatment within 7 days of their exposure. Treatment consists of taking the mentioned antibiotics for at least 7 days.[8]
A formalin inactivated vaccination is available for adults who had a higher chance of contracting the disease. However, its effectiveness is limited and it can cause severe inflammation. Scientists are currently experimenting with a recombinant fusion protein of the Yersinia pestis F1 and V antigens. Natural or induced immunity can be achieved by the production of specific opsonic antibodies against F1 and V antigens. However bacteria that lack the F1 antigen are still virulent, and the V antigens are sufficiently variable, so that vaccines composed of these antigens may not be fully protective against all strains of the bacteria.[11]
Current Research
Plague research is being conducted by several government agencies in an effort to help in the diagnosis, treatment, and prevention caused by Yersinia pestis, as well as addressing the need to defend against deliberately caused disease outbreaks. Specifically this research focuses on developing a vaccine against the pneumonic plague, developing antibiotics to prevent and treat infection, and most importantly studying and identifying genes and proteins in Yersinia pestis that infect the digestive tract of fleas and enable them to grow and function in humans.[4]
Specifically, scientists at the National Institute of Allergy and Infectious Diseases (NIAID) Rocky Mountain Laboratories (RML), found that three genes in Yersinia pestis change it from a harmless, long-term inhabitant in the flea’s mid gut to one that migrates and accumulates in its foregut. As a result of this change, the flea begins to starve, causing it to fanatically feed, during which it regurgitates the bacteria and hence transmits the plague. Although it was known for quite some time that the bacterium’s transmission is dependant on the fleas as hosts, there was little understanding about the molecular and genetic mechanisms by which this colonization occurs. They began experiments on three hemin storage genes (hms), which are abundant in red blood cells, and acts as the iron-containing part of the hemoglobin molecule that binds oxygen. To understand the role of these genes in the host Dr. Hinnebusch conducted experiments with oriental rat fleas, in which he injected the normal Yersinia pestis bacteria and a mutant form which was missing the hms genes. After four weeks, the scientists found that only those fleas infected with the normal bacteria developed the foregut blockage, which was accompanied by a high rate of mortality. These results indicated that the hms genes are required for Yersinia pestis to cause the foregut blockage.[4]
Next, the scientists highlighted both forms of Yersinia pestis with fluoresce green and after dissecting the host fleas, they noted how the mutant bacteria remained in the midgut while the normal bacteria had migrated to the foregut in many fleas which eventually, became packed with bacteria. Now other genes are being studied that may affect the bacteria’s ability to transmit infection and it was observed that the blockage that develops in the flea foregut breaks down at temperatures above 26.66 °C (80 °F) to 29.44 °C (85 °F). Scientists are trying to determine why this occurs and if such temperature changes might suppress the products of hms or other genes.[4]
References
- ↑ Yersinia pestis (Plague), University of Iowa Hygienic Laboratory, 2008, Former Reference 9
- ↑ 2.0 2.1 Parkhill J et al. (2001 Oct 4), "Genome sequence of Yersinia pestis, the causative agent of plague", Nature 413 (6855): 523-7, Former example 1
- ↑ 3.0 3.1 Prentice, Mike, Yersinia pestis (plague, Black Death, Bubonic Plague), Former example 6
- ↑ 4.0 4.1 4.2 4.3 Doepel, Laurie K. (July 18, 1996), Scientists Identify Genes Critical to Transmission of Bubonic Plague, National Institute of Allergy and Infectious Diseases (NIAID), former citation 4
- ↑ 5.0 5.1 5.2 Chamberlain, Neil R. (8/3/2004.), Plague, Former reference 10
- ↑ 6.0 6.1 Schoenstadt, Arthur (October 14, 2006), Yersinia Pestis, former example 2
- ↑ Minnaganti, Venkat R. (May 12, 2006), "Plague", eMedicine, former citation 5
- ↑ 8.0 8.1 8.2 8.3 Bellazzini, Marc A; Myint, Michael (March 2006), "Yersinia Pestis: New Challenges in the Age of Bioterrorism”", Resident and Staff 56 (3), Former reference 7
- ↑ Coordinating Center for Infectious Diseases (CCID). National Center for Zoonotic, Vector-Borne, and Enteric Diseases (NCZVED) Division of Vector-Borne Infectious Diseases (April 5, 2005), Frequently Asked Questions about Plague
- ↑ 10.0 10.1 10.2 10.3 10.4 Smith, Christine A. (1996), Plague in the Ancient World: A Study from Thucydides to Justinian, Former reference 11
- ↑ Gage, Kenneth L, Dennis, David T, Tsai, Theodore F. (9/9/1998), Prevention of Plague: Recommendations of the Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control