Radiochemistry
Radiochemistry is the chemistry of radioactive materials, where radioactive isotopes of elements are used to study the properties and chemical reactions of non-radioactive isotopes (often within radiochemistry the absence of radioactivity leads to a substance being described as being inactive as the isotopes are stable). Much of radiochemistry deals with the use of radioactivity to study ordinary chemical reactions. Radiochemistry includes the study of both natural and man-made radioisotopes. Many naturally occurring substances contain radioactive elements including uranium (U), radium (Ra) and thorium (Th).
Main decay modes
All radioisotopes are unstable isotopes of elements—undergo nuclear decay and emit some form of radiation. The radiation emitted can be one of three types, called alpha, beta, or gamma radiation.
1. (alpha) radiation - the emission of an alpha particle, which contains two protons and two neutrons, from an atomic nucleus. When this occurs, the atom’s atomic mass decreases by 4 units and atomic number decreases by 2.
2. (beta) radiation - the transmutation of a neutron into an electron and a proton. After this happens, the electron is emitted from the nucleus into the electron cloud.
3. (gamma) radiation - the emission of electromagnetic energy (such as X-rays) from the nucleus of an atom. This usually occurs during alpha or beta radioactive decay.
These three types of radiation can distinguished by their difference in penetrating power.
Alpha radiation can be blocked quite easily by a few centimetres in air or a piece of paper, and is equivalent to a helium nucleus. Beta radiation can be blocked by an aluminium sheet just a few millimetres thick and are electrons. Gamma radiation is the most penetrating, and is a massless chargeless high energy photon. Gamma radiation requires an appreciable amount of heavy metal radiation shielding (usually lead or barium-based) to reduce its intensity.
Activation analysis
By neutron irradiation of objects it is possible to induce radioactivity, and this activation of stable isotopes to create radioisotopes is the basis of neutron activation analysis. One of the most interesting objects which has been studied in this way is the hair of Napoleon's head, which have been examined for their arsenic content.[1]
A series of different experimental methods have been designed to enable the measurement of different elements in different matrices. To reduce the effect of the matrix it is common to use the chemical extraction of the wanted element and/or to allow the radioactivity due to the matrix elements to decay before the measurement of the radioactivity.
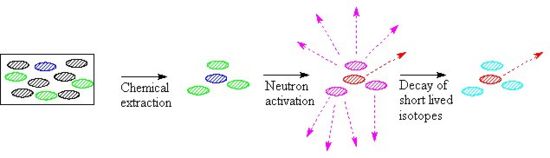
The effects of a series of different cooling times can be seen if a hypothetical sample which contains sodium, uranium and cobalt in a 100:10:1 ratio is subjected to a very short pulse of thermal neutrons. In the following bar chart the radioactivity due to each of these three elements is shown, note that the uranium-239 decays very quickly to neptunium 239Np, which decays with a halflife of 2.36 days.
Biochemical uses
One biological application is the study of DNA using radioactive phosphorus (32P). In these experiments, stable phosphorus is replaced by the chemical identical radioactive P-32, and the resulting radioactivity is used in analysis of the molecules and their behaviour.
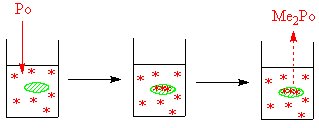
Another example is the work done on the methylation of elements such as sulphur, selenium, tellurium and polonium by living organisms. Bacteria can convert these elements into volatile compounds,[2] it is thought that methylcobalamin (vitamin B12 alkylates these elements to create the dimethyls. A combination of Cobaloxime and inorganic polonium in sterile water forms a volatile polonium compound, while a control experiment which did not contain the cobalt compound did not form the volatile polonium compound.[3]. For the sulphur work the isotope 35S was used, while for polonium 207Po was used. In some related work by the addition of 57Co to the bacterial culture, followed by isolation of the cobalamin from the bacteria (and the measurement of the radioactivity of the isolated cobalamin) it was shown that the bacteria convert available cobalt into methylcobalamin.
Environmental
Radiochemistry also includes the study of the behaviour of radioisotopes in the environment; for instance, a forest or grass fire can make radioisotopes become mobile again.[4] In these experiments, fires were started in the exclusion zone around Chernobyl and the radioactivity in the air downwind was measured.
It is important to note that a vast number of processes can release radioactivity into the environment, for example the action of cosmic rays on the air is responsible for the formation of radioisotopes (such as 14C and 32P), the decay of 226Ra forms 222Rn which is a gas which can diffuse through rocks before entering buildings[5][6][7] and dissolve in water and thus enter drinking water[8] in addition human activities such as bomb tests, accidents(for example [9]) and normal releases from industry have resulted in the release of radioactivity.
Chemical form of the actinides
The environmental chemistry of some radioactive elements such as plutonium is complicated by the fact that solutions of this element can undergo disproportionation[10] and as a result many different oxidation states can coexist at once. Some work has been done on the identification of the oxidation state and coordination number of plutonium and the other actinides under different conditions has been done.[4] This includes work on both solutions of relatively simple complexes[11] and work on colloids [12] Two of the key matrices are soil/rocks and concrete, in these systems the chemical properties of plutonium have been studied using methods such as EXAFS and XANES.[13][5][6]
The thermodynamics of the actinides controls much of their environmental chemistry, a series of reviews on uranium and the other actinides has been published by the NEA. [14]
Movement of colloids
While binding of a metal to the surfaces of the soil particles can prevent its movement through a layer of soil, it is possible for the particles of soil which bear the radioactive metal can migrate as colloidal particles through soil. This has been shown to occur using soil particles labelled with 134Cs, these have been shown to be able to move through cracks in the soil.[15]
Normal background
Radioactivity is present everywhere (and has been since the formation of the earth). According to the IAEA, one kilogram of soil typically contains the following amounts of the following three natural radioisotopes 370 Bq 40K (typical range 100-700 Bq), 25 Bq 226Ra (typical range 10-50 Bq), 25 Bq 238U (typical range 10-50 Bq) and 25 Bq 232Th (typical range 7-50 Bq).[16]
Action of microorganisms
The action of microorganisms can fix uranium; it is interesting to note that Thermoanaerobacter can used chromium(VI), iron(III), cobalt(III), manganese(IV) and uranium(VI) as electron acceptors while acetate, glucose, hydrogen, lactate, pyruvate, succinate, and xylose can act as electron donors for the metabolism of the bacteria. In this way the metals can be reduced to form magnetite (Fe3O4), siderite (FeCO3), rhodochrosite (MnCO3), and uraninite (UO2).[17] Other researchers have also worked on the fixing of uranium using bacteria[7][8][9], Livens FR et. al. (Working at Manchester) have suggested that the reason why Geobacter sulfurreducens can reduce UO22+ carions to uranium dioxide is that the bacteria reduce the uranyl cations to UO2+ which then undergoes disproportionation to form UO22+ and UO2. This reasoning was based (at least in part) on the observation that NpO2+ is not converted to an insoluble neptunium oxide by the bacteria.[18]
References
- ↑ Smith H et al. (1962) Nature 194:725-6.
- ↑ Momoshima N, Li-X. Song, Osaki S, Maeda Y (2002) Biologically induced Po emission from fresh water. J Envir Radioactivity 63:187-97.
- ↑ Momoshima N, Li-X. Song, Osaki S, Maeda Y (2001) Formation and emission of volatile polonium compound by microbial activity and polonium methylation with methylcobalamin. Envir Sci Technol, 35:2956-60.
- ↑ Yoschenko VI et al (2006) Resuspension and redistribution of radionuclides during grassland and forest fires in the Chernobyl exclusion zone: part I. Fire experiments J Envir Radioact 86:143-63 PMID 16213067
- ↑ Janja Vaupotič and Ivan Kobal, "Effective doses in schools based on nanosize radon progeny aerosols", Atmospheric Environment, 2006, 40, 7494-7507
- ↑ Michael Durand, Building and Environment, "Indoor air pollution caused by geothermal gases", 2006, 41, 1607-1610
- ↑ Boffetta P "Human cancer from environmental pollutants: The epidemiological evidence", Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2006, 608, 157-162
- ↑ Forte M et al. The measurement of radioactivity in Italian drinking waters. Microchemical J, 2007, 85:98-102
- ↑ Pöllänen R et al. "Multi-technique characterization of a nuclear bomb particle from the Palomares accident", J Envir Radioactivity, 2006, 90:15-28
- ↑ Rabideau, S.W., J Am Chem Society, 1957, 79, 6350-6353
- ↑ Allen PG et al. Investigation of aquo and chloro complexes of UO22+, NpO2+, Np4+, and Pu3+ by X-ray absorption fine structure spectroscopy. Inorganic Chemistry, 1997, 36, 4676-4683
- Clark DL et al. (1998) Identification of the limiting species in the plutonium(IV) carbonate system. Solid state and solution molecular structure of the [Pu(CO3)5]6- Ion. Inorganic Chemistry 37:2893-9
- ↑ Rothe J et al. XAFS and LIBD investigation of the formation and structure of colloidal Pu(IV) hydrolysis products. Inorganic Chem, 2004, 43:4708-18
- ↑ M. C. Duff, et al. (1999) "Mineral associations and average oxidation states of sorbed Pu on Tuff. Environ Sci Technol33:2163-9.
- ↑ For a review on americium and uranium see [1] and [2]
- ↑ Whicker RD, Ibrahim SA (2006) Vertical migration of 134Cs bearing soil particles in arid soils: implications for plutonium redistribution", J Envir Radioactivity 88:171-88.
- ↑ Generic Procedures for Assessment and Response during a Radiological Emergency, IAEA TECDOC Series number 1162, published in 2000 [3]
- ↑ Roh Y et al. (2002) Isolation and Characterization of Metal-Reducing Thermoanaerobacter Strains from Deep Subsurface Environments of the Piceance Basin, Colorado. Applied and Environmental Microbiology 68:6013-20.
- ↑ Renshaw JC et al. (2005) Environ Sci Technol 39:5657-60.