Thalidomide: Difference between revisions
imported>Howard C. Berkowitz (New page: '''Thalidomide''' is a biologic response modifier with an increasing number of applications in severe inflammatory, immune, or dermatologic diseases. It is anti-inflammatory, anti-angi...) |
John Leach (talk | contribs) m (Text replacement - "biological response modifier" to "biological response modifier") |
||
| (12 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
'''Thalidomide''' is a | {{subpages}} | ||
{{TOC|right}} | |||
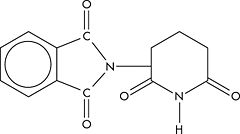
[[Image:Thalidomide.jpg|left|thumb|240px|Structure of thalidomide]] | |||
'''Thalidomide''' is a biological response modifier with an increasing number of applications in severe inflammatory, immune, or dermatologic diseases. It is anti-inflammatory, anti-angiogenic, and immulomodulatory. A piperidinyl isoindole originally introduced as a non-barbiturate hypnotic in the 1950s, it causes extreme fetal malformations or fetal death, and was unavailable for many years.<ref>{{MeSH}}</ref> | |||
{| " style="border: solid; center; width:450px;" | |||
!align=left colspan=3 style="background:#{{{color}}}"| Thalidomide is absolutely contraindicated in pregnancy. Female patients who receive it <u>must</u> use two forms of contraception or abstain completely from sexual intercourse. | |||
Men taking thalidomide also must use contraception, because it is not known if the birth defects can be passed in sperm. | |||
Patients must not donate blood for at least one year after discontinuing thalidomide therapy.<ref>{{citation | |||
| url = http://cerhr.niehs.nih.gov/common/thalidomide.html | |||
| title = Thalidomide | |||
| publisher = National Toxicology, [[U.S. Department of Health and Human Services]] | |||
}}</ref> | |||
|- | |||
|} | |||
Physicians are cautiously approaching it for a number of disorders.<ref>{{citation | |||
| url = http://news.bbc.co.uk/2/hi/americas/134329.stm | |||
| date = 17 July 1998 | |||
| title = Fresh start for notorious drug | |||
| journal = BBC News | |||
}}</ref> Even though its mechanisms have not been fully characterized, it has a number of unique features.<ref>{{citation | |||
| journal = Postgrad Med J | year = 2003| volume = 79 | pages = 127-132 | |||
| doi=10.1136/pmj.79.929.127 | |||
| title = Review: Thalidomide and its derivatives: emerging from the wilderness | |||
| author = Gordon JN, Goggin PM | |||
| url = http://pmj.bmj.com/content/79/929/127.full | |||
}}</ref> | |||
==Indications== | ==Indications== | ||
It was reintroduced for the narrow indication of preventing and treating severe skin reactions in [[leprosy]], and for [[multiple myeloma]]. Approved usages as an [[orphan drug]] extended to [[AIDS wasting syndrome]], prevention and treatment of severe recurrent [[aphthous stomatitis]] in severely, terminally immunocompromised patients; prevention and treatment of [[graft-versus-host disease]] in patients receiving bone marrow transplantation; treatment of clinical manifestations of both tuberculous and nontuberculous mycobacterial infection; treatment of [[Crohn’s disease]]; and treatment of primary brain tumors. | |||
In addition, thalidomide has been used for the treatment of a variety of inflammatory and/or dermatologic disorders, treatment of various HIV-associated conditions, and treatment of various malignancies.<ref>{{citation | |||
| url = http://www.medscape.com/druginfo/monograph?cid=med&drugid=16389&drugname=Thalidomide+Oral&monotype=monograph&secid=2 | |||
| title = Monograph: Thalidomide — Uses | |||
| publisher = American Society of Health System Pharmacists/Medscape | |||
}}</ref> | |||
It has been used in [[Behcet's Syndrome]],<ref>{{citation | |||
| title = Thalidomide in the Treatment of the Mucocutaneous Lesions of the Behcet Syndrome | |||
A Randomized, Double-Blind, Placebo-Controlled Trial | |||
| author = Vedat Hamuryudan, ''et al.'' | |||
| journal = Ann Intern Med | |||
| date = 15 March 1998 | volume = 128 | issue = 6 | pages = 443-450 | |||
| url = http://www.annals.org/content/128/6/443.full}}</ref> which occasioned a discussion of risks and benefits.<ref>{{citation | |||
| title = Editorial: Behcet Disease and the Emergence of Thalidomide | |||
| author =George E. Ehrlich | |||
| journal = Ann Intern Med | |||
| date = 15 March 1998 | volume = 128 | issue =6 | pages = 494-495 | |||
| url = http://www.annals.org/content/128/6/494.full | |||
}}</ref> | |||
==Pharmacology== | ==Pharmacology== | ||
While all of its mechanisms are not fully understood, its functions include:<ref>{{citation | While all of its mechanisms are not fully understood, its functions include:<ref>{{citation | ||
| http://www.medscape.com/druginfo/monograph?cid=med&drugid=16389&drugname=Thalidomide+Oral&monotype=monograph&secid=8 | | url = http://www.medscape.com/druginfo/monograph?cid=med&drugid=16389&drugname=Thalidomide+Oral&monotype=monograph&secid=8 | ||
| title = Monograph: Thalidomide — Pharmacology | | title = Monograph: Thalidomide — Pharmacology | ||
| publisher = American Society of Health System Pharmacists/Medscape | | publisher = American Society of Health System Pharmacists/Medscape | ||
| Line 17: | Line 61: | ||
*Possible suppression of macrophage involvement of prostaglandin synthesis and modulation of [[interleukin-10]] and [[interleukin-12]] production by peripheral blood [[monocyte]]s. | *Possible suppression of macrophage involvement of prostaglandin synthesis and modulation of [[interleukin-10]] and [[interleukin-12]] production by peripheral blood [[monocyte]]s. | ||
While its anti-inflammatory and immunomodulatory effects are complex, they appear different from those of other agents, including [[corticosteroid]]s, cyclosporin (e.g., [[cyclosporine]]) or macrolide (e.g., [[tacrolimus]]) immunosuppressants, [[pentoxifylline]], immunosuppressive purine analogs (e.g. [[azathioprine]]) and purine metabolism inhibitors (e.g., [[mycophenolic acid]]), and | While its anti-inflammatory and immunomodulatory effects are complex, they appear different from those of other agents, including [[corticosteroid]]s, cyclosporin (e.g., [[cyclosporine]]) or macrolide (e.g., [[tacrolimus]]) immunosuppressants, [[pentoxifylline]], immunosuppressive purine analogs (e.g. [[azathioprine]]) and purine metabolism inhibitors (e.g., [[mycophenolic acid]]), and [[Non-steroidal anti-inflammatory agent]]s. | ||
It does not appear to interfere with antimicrobial defense mechanisms, | It does not appear to interfere with antimicrobial defense mechanisms, lymphocyte proliferation, granuloma formation, and delayed hypersensitivity reactions. | ||
==References== | ==References== | ||
{{reflist}} | {{reflist|2}} | ||
Latest revision as of 02:45, 21 March 2024
Thalidomide is a biological response modifier with an increasing number of applications in severe inflammatory, immune, or dermatologic diseases. It is anti-inflammatory, anti-angiogenic, and immulomodulatory. A piperidinyl isoindole originally introduced as a non-barbiturate hypnotic in the 1950s, it causes extreme fetal malformations or fetal death, and was unavailable for many years.[1]
| Thalidomide is absolutely contraindicated in pregnancy. Female patients who receive it must use two forms of contraception or abstain completely from sexual intercourse.
Men taking thalidomide also must use contraception, because it is not known if the birth defects can be passed in sperm. Patients must not donate blood for at least one year after discontinuing thalidomide therapy.[2] |
|---|
Physicians are cautiously approaching it for a number of disorders.[3] Even though its mechanisms have not been fully characterized, it has a number of unique features.[4]
Indications
It was reintroduced for the narrow indication of preventing and treating severe skin reactions in leprosy, and for multiple myeloma. Approved usages as an orphan drug extended to AIDS wasting syndrome, prevention and treatment of severe recurrent aphthous stomatitis in severely, terminally immunocompromised patients; prevention and treatment of graft-versus-host disease in patients receiving bone marrow transplantation; treatment of clinical manifestations of both tuberculous and nontuberculous mycobacterial infection; treatment of Crohn’s disease; and treatment of primary brain tumors.
In addition, thalidomide has been used for the treatment of a variety of inflammatory and/or dermatologic disorders, treatment of various HIV-associated conditions, and treatment of various malignancies.[5]
It has been used in Behcet's Syndrome,[6] which occasioned a discussion of risks and benefits.[7]
Pharmacology
While all of its mechanisms are not fully understood, its functions include:[8]
- immunomodulator for tumor necrosis factor-alpha
- costimulatory or adjuvant effect on T-lymphocytes resulting in increased T-cell proliferation and increased production of interleukin-2 and interferon gamma
- modulation of leukocyte migration and chemotaxis.
- Possible suppression of macrophage involvement of prostaglandin synthesis and modulation of interleukin-10 and interleukin-12 production by peripheral blood monocytes.
While its anti-inflammatory and immunomodulatory effects are complex, they appear different from those of other agents, including corticosteroids, cyclosporin (e.g., cyclosporine) or macrolide (e.g., tacrolimus) immunosuppressants, pentoxifylline, immunosuppressive purine analogs (e.g. azathioprine) and purine metabolism inhibitors (e.g., mycophenolic acid), and Non-steroidal anti-inflammatory agents.
It does not appear to interfere with antimicrobial defense mechanisms, lymphocyte proliferation, granuloma formation, and delayed hypersensitivity reactions.
References
- ↑ Anonymous (2024), Thalidomide (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ Thalidomide, National Toxicology, U.S. Department of Health and Human Services
- ↑ "Fresh start for notorious drug", BBC News, 17 July 1998
- ↑ Gordon JN, Goggin PM (2003), "Review: Thalidomide and its derivatives: emerging from the wilderness", Postgrad Med J 79: 127-132, DOI:10.1136/pmj.79.929.127
- ↑ Monograph: Thalidomide — Uses, American Society of Health System Pharmacists/Medscape
- ↑ Vedat Hamuryudan, et al. (15 March 1998), "[http://www.annals.org/content/128/6/443.full Thalidomide in the Treatment of the Mucocutaneous Lesions of the Behcet Syndrome A Randomized, Double-Blind, Placebo-Controlled Trial]", Ann Intern Med 128 (6): 443-450
- ↑ George E. Ehrlich (15 March 1998), "Editorial: Behcet Disease and the Emergence of Thalidomide", Ann Intern Med 128 (6): 494-495
- ↑ Monograph: Thalidomide — Pharmacology, American Society of Health System Pharmacists/Medscape