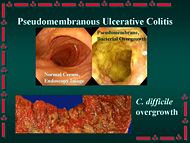
Pseudomembranous enterocolitis
| Pseudomembranous enterocolitis | |
|---|---|
| Pseudomembranous enterocolitis | |
| MeSH | D004761 |
In gastroenterology, pseudomembranous enterocolitis is an "acute inflammation of the intestinal mucosa that is characterized by the presence of pseudomembranes or plaques in the small intestine (pseudomembranous enteritis) and the large intestine (pseudomembranous colitis). It is commonly associated with antibiotic therapy and clostridium difficile colonization."[1] The disorder is an increasing matter of concern, as it is one of the more common nosocomial infections, but is also being seen in community-acquired cases.
Epidemiology
About 50% of patients with diarrhea after antibiotics that is severe enough to be admitted to the hospital have pseudomembranes on colonoscopy.[2]
Diagnosis
Clinical practice guidelines address diagnosis.[3]
Sigmoidoscopy has a sensitivity of 31% in detecting pseudomembranes as compared to colonosopy.[4]
Treatment
Clinical practice guidelines are available to direct management.[3]
Antibiotics
Various antibiotics have been studied in randomized controlled trials.[5][6][7] Teicoplanin may be the most effective antibiotic.[5][6] Vancomycin has an insignificant trend towards being better than metronidazole;[5] metronidazole is likely to be the least expensive of the group and is not a last resort for other highly resistant organisms.
"Our findings suggest that metronidazole and vancomycin are equally effective for the treatment of mild CDAD, but vancomycin is superior for treating patients with severe CDAD." according to a randomized controlled trial. [8]
Consider vancomycin if the patient has two or more points from the following:[9]
- 1 point for each of
- WBC > 15k
- albumin < 2.5
- age > 60
- temperature > 38.3° C
- 2 points for each of
- Pseudomembranous colitis
- Intensive care
It should be noted that vancomycin is given orally in this condition, and is not absorbed systemically. While it still needs to be used with care to avoid the development of resistant organisms, the risk may be lower than if it is used as a parenteral therapy for already resistant pathogens such as methicillin-resistant Staphylococcus aureus.
In a randomized controlled trial, the relative risk ratio of fidaxomicin, as compared to vancomycin, for recurrence of C. difficile infection was 0.6 and the relative risk reduction was 40.0%. In populations similar to those in this study which had a rate of risk as measured by the recurrence of C. difficile infection of 25% without treatment, the number needed to treat is 10. [10]
A case serioes suggests tigecycline for severe disease.[11]
Administration of bacteria
Probiotic administration may[12] or may not [13]help according to a meta-analyses and a more recent randomized controlled trial.[14] However, probiotics can be harmful among intensive care patients.[15]
Monoclonal antibodies
In a randomized controlled trial, the relative risk ratio of adjunct treatment with monoclonal antibodies against C. difficile toxins, as compared to placebo, for recurrence of C. difficile infection was 0.3 and the relative risk reduction was 72.0%. In populations similar to those in this study which had a rate of risk as measured by the recurrence of C. difficile infection of 25% without treatment, the number needed to treat is 6. [16]
Recurrent infection
A clinical prediction rule found that recurrent infection is more likely if age is more than 65 years, the patient has severe or fulminant illness, and additional antibiotic exposure occurs after after treatment of the initial Clostridium difficile infection.[17] Use of antacids may also be a risk factor for recurrence.[18]
Asymptomatic carriage should not be treated according to a meta-analysis.[19]
Antibiotics
| Trial | Patients | Intervention | Comparison | Outcome | Results - cure | Results - recurrence | ||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |||||
| Louie et al[10] 2011 |
629 patients • mild to severe |
Fidaxomicin • 200 mg twice daily • 10 days |
Vancomycin • 125 mg 4 times/day • 10 days |
stool toxin after 4 weeks | 88% | 86% | 15% | 25% |
| Zar et al[8] 2007 |
150 patients • mild to severe |
Metronidazole • 250 mg 4 times/day • 10 days |
Vancomycin • 125 mg 4 times/ day • 10 days |
stool toxin after 3 weeks | 90% (76% if severe) |
98% (97% if severe) |
15% | 14% |
Observational studies conflict regarding the best agent and suggest vancomycin may[20] or may not[21] be better than metronidazole. Various methods exist for the administration of vancomycin[20][22] and metronidazole[23].
| Patients | Intervention / duration | Comparison | Outcome: Recurrence rate | |
|---|---|---|---|---|
| Approximately 2 weeks | ||||
| Vancomycin constant dose[21] | 171 patients | 0.5 to 2 grams daily 10 - 14 days |
NA | 40% |
| Metronidazole constant dose[21] | 115 patients | 1.0 to 1.5 grams per day 10 - 14 days |
NA | 37% |
| Metronidazole constant dose[23] | 20 patients | Metronidzole 1200 mg daily 14 days |
NA | 45% |
| Vancomycin constant dose[20] | 83 patients | 0.5 to 3 grams/day 10 - 16 days |
NA | 54% |
| high dose[20] | 21 patients | ≥2 grams/day 10 - 16 days |
NA | 43% |
| medium dose[20] | 14 patients | 1 - 2 grams/day 10 - 16 days |
NA | 71% |
| low dose[20] | 48 patients | < 1 grams/day 10 - 16 days |
NA | 54% |
| Metronidazole constant dose[20] | 36 patients | 0.5 to 3 grams/day 10 - 16 days |
NA | 42% |
| Approximately 3 weeks | ||||
| Vancomycin tapered dose[20] | 29 patients | Varying doses 21.5 ± 10.0 days |
NA | 31% |
| Vancomycin pulsed dose[20] | 7 patients | Varying doses 21 days |
NA | 14% |
| More than 3 weeks | ||||
| Vancomycin followed by rifaximin[24] | 8 patients | Vancomycin of unknown duration followed by rifaximin 400–800 mg daily for 14 days ≥ 21 days |
NA | 13% |
| Vancomycin tapered & pulsed dose[22] | 22 patients | 125 mg four times daily tapered to 125 mg every third day 42 days |
NA | 0% |
Serial therapy with vancomycin and rifaximin has been studied in a small uncontrolled series of patients.[24]
Administration of bacteria
Probiotics may help.[25] However, probiotics can be harmful among intensive care patients.[15]
Rectal infusion of feces helped according to case reports.[26][27]
References
- ↑ Anonymous (2024), Pseudomembranous enterocolitis (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ Lee KS, Shin WG, Jang MK, et al (October 2006). "Who are susceptible to pseudomembranous colitis among patients with presumed antibiotic-associated diarrhea?". Dis. Colon Rectum 49 (10): 1552–8. DOI:10.1007/s10350-006-0694-z. PMID 17028914. Research Blogging.
- ↑ 3.0 3.1 Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC et al. (2010). "Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA).". Infect Control Hosp Epidemiol 31 (5): 431-55. DOI:10.1086/651706. PMID 20307191. Research Blogging.
- ↑ Seppala, K, Hjelt, L, Supponen, P. Colonoscopy in the diagnosis of antibiotic-associated colitis. Scand J Gastroenterol 1981; 16:465. PMID 7323683
- ↑ 5.0 5.1 5.2 Nelson R (2007). "Antibiotic treatment for Clostridium difficile-associated diarrhea in adults". Cochrane Database Syst Rev (3): CD004610. DOI:10.1002/14651858.CD004610.pub3. PMID 17636768. Research Blogging.
- ↑ 6.0 6.1 Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W (May 1996). "Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea". Clin. Infect. Dis. 22 (5): 813–8. PMID 8722937. [e]
- ↑ Musher DM, Logan N, Bressler AM, Johnson DP, Rossignol JF (January 2009). "Nitazoxanide versus Vancomycin in Clostridium difficile Infection: A Randomized, Double-Blind Study". Clin. Infect. Dis.. DOI:10.1086/596552. PMID 19133801. Research Blogging.
- ↑ 8.0 8.1 8.2 Zar FA, Bakkanagari SR, Moorthi KM, Davis MB (2007). "A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity.". Clin Infect Dis 45 (3): 302-7. DOI:10.1086/519265. PMID 17599306. Research Blogging.
- ↑ Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007 Aug 1;45(3):302-7. PMID 17599306
- ↑ 10.0 10.1 10.2 Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y et al. (2011). "Fidaxomicin versus vancomycin for Clostridium difficile infection.". N Engl J Med 364 (5): 422-31. DOI:10.1056/NEJMoa0910812. PMID 21288078. Research Blogging.
- ↑ Herpers BL, Vlaminckx B, Burkhardt O, et al. (June 2009). "Intravenous tigecycline as adjunctive or alternative therapy for severe refractory Clostridium difficile infection". Clin. Infect. Dis. 48 (12): 1732–5. DOI:10.1086/599224. PMID 19435431. Research Blogging.
- ↑ McFarland LV (April 2006). "Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease". Am. J. Gastroenterol. 101 (4): 812–22. DOI:10.1111/j.1572-0241.2006.00465.x. PMID 16635227. Research Blogging.
- ↑ Pillai A, Nelson R (2008). "Probiotics for treatment of Clostridium difficile-associated colitis in adults". Cochrane Database Syst Rev (1): CD004611. DOI:10.1002/14651858.CD004611.pub2. PMID 18254055. Research Blogging.
- ↑ Klarin B, Wullt M, Palmquist I, Molin G, Larsson A, Jeppsson B (September 2008). "Lactobacillus plantarum 299v reduces colonisation of Clostridium difficile in critically ill patients treated with antibiotics". Acta Anaesthesiol Scand 52 (8): 1096–102. DOI:10.1111/j.1399-6576.2008.01748.x. PMID 18840110. Research Blogging.
- ↑ 15.0 15.1 Muñoz P, Bouza E, Cuenca-Estrella M, et al (June 2005). "Saccharomyces cerevisiae fungemia: an emerging infectious disease". Clin. Infect. Dis. 40 (11): 1625–34. DOI:10.1086/429916. PMID 15889360. Research Blogging.
- ↑ Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN et al. (2010). "Treatment with monoclonal antibodies against Clostridium difficile toxins.". N Engl J Med 362 (3): 197-205. DOI:10.1056/NEJMoa0907635. PMID 20089970. Research Blogging.
- ↑ Hu MY, Katchar K, Kyne L, et al (December 2008). "Prospective Derivation and Validation of a Clinical Prediction Rule for Recurrent Clostridium difficile Infection". Gastroenterology. DOI:10.1053/j.gastro.2008.12.038. PMID 19162027. Research Blogging.
- ↑ Garey KW, Sethi S, Yadav Y, DuPont HL (December 2008). "Meta-analysis to assess risk factors for recurrent Clostridium difficile infection". J. Hosp. Infect. 70 (4): 298–304. DOI:10.1016/j.jhin.2008.08.012. PMID 18951661. Research Blogging.
- ↑ Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN (February 1998). "Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea". Lancet 351 (9103): 633–6. DOI:10.1016/S0140-6736(97)08062-8. PMID 9500319. Research Blogging.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 20.8 McFarland LV, Elmer GW, Surawicz CM (July 2002). "Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease". Am. J. Gastroenterol. 97 (7): 1769–75. DOI:10.1016/S0002-9270(02)04195-3. PMID 12135033. Research Blogging.
- ↑ 21.0 21.1 21.2 Pépin J, Routhier S, Gagnon S, Brazeau I (March 2006). "Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada". Clin. Infect. Dis. 42 (6): 758–64. DOI:10.1086/501126. PMID 16477549. Research Blogging.
- ↑ 22.0 22.1 Tedesco FJ, Gordon D, Fortson WC (November 1985). "Approach to patients with multiple relapses of antibiotic-associated pseudomembranous colitis". Am. J. Gastroenterol. 80 (11): 867–8. PMID 4050760. [e]
- ↑ 23.0 23.1 Mattila E, Anttila VJ, Broas M, et al (2008). "A randomized, double-blind study comparing Clostridium difficile immune whey and metronidazole for recurrent Clostridium difficile-associated diarrhoea: efficacy and safety data of a prematurely interrupted trial". Scand. J. Infect. Dis. 40 (9): 702–8. PMID 19086244. [e]
- ↑ 24.0 24.1 Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN (March 2007). "Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin". Clin. Infect. Dis. 44 (6): 846–8. DOI:10.1086/511870. PMID 17304459. Research Blogging.
- ↑ Surawicz CM (July 2008). "Role of probiotics in antibiotic-associated diarrhea, Clostridium difficile-associated diarrhea, and recurrent Clostridium difficile-associated diarrhea". J. Clin. Gastroenterol. 42 Suppl 2: S64–70. DOI:10.1097/MCG.0b013e3181646d09. PMID 18545161. Research Blogging.
- ↑ Schwan A, Sjölin S, Trottestam U, Aronsson B (October 1983). "Relapsing clostridium difficile enterocolitis cured by rectal infusion of homologous faeces". Lancet 2 (8354): 845. PMID 6137662. [e]
- ↑ Nieuwdorp M, van Nood E, Speelman P, et al (August 2008). "[Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces]" (in Dutch; Flemish). Ned Tijdschr Geneeskd 152 (35): 1927–32. PMID 18808083. [e]