Inositol: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk (stub for letter week) |
imported>David E. Volk mNo edit summary |
||
| Line 3: | Line 3: | ||
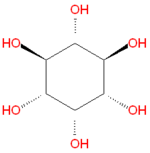
{{Image|Myo-inositol.png|right|150px|Myo-inositol, the most common inositol [[stereoisomer]].}} | {{Image|Myo-inositol.png|right|150px|Myo-inositol, the most common inositol [[stereoisomer]].}} | ||
'''Inositol''' refers to a collection [[hexose]] [[carbohydrate]]s based on a central [[cyclohexane]] structure with six [[ | '''Inositol''' refers to a collection [[hexose]] [[carbohydrate]]s based on a central [[cyclohexane]] structure with six [[hydroxyl]] (OH) groups. Although myo-inositol, previously called meso-inositol, is the most common version found in nature, a number of other stereoisomeric forms are produced naturally. Inositol acts as a [[fatty acid]] transport by coupling its hydroxyl oxygen atoms with the carboxyl groups of fatty acids. The stereochemistry of myo-inositol is denoted by the [[IUPAC]] name cis-1,2,3,5-trans-4,6-cyclohexanehexol. | ||
Revision as of 09:52, 11 March 2011
Inositol refers to a collection hexose carbohydrates based on a central cyclohexane structure with six hydroxyl (OH) groups. Although myo-inositol, previously called meso-inositol, is the most common version found in nature, a number of other stereoisomeric forms are produced naturally. Inositol acts as a fatty acid transport by coupling its hydroxyl oxygen atoms with the carboxyl groups of fatty acids. The stereochemistry of myo-inositol is denoted by the IUPAC name cis-1,2,3,5-trans-4,6-cyclohexanehexol.