Electric dipole: Difference between revisions
imported>Paul Wormer |
imported>Paul Wormer |
||
| Line 2: | Line 2: | ||
In [[chemistry]], an '''electric dipole''' is a charge distribution consisting of two electric point charges of opposite sign and equal magnitude. | In [[chemistry]], an '''electric dipole''' is a charge distribution consisting of two electric point charges of opposite sign and equal magnitude. | ||
Often | Often it is added to this definition that the distance ''r'' between the two point charges is small—if this is the case the dipole is a '''point dipole'''. ''Small'' is a relative concept and requires further specification. In general one is interested in the electric potential field due to the dipolar charge distribution at a distance ''R'' from the midpoint of the dipole. If it holds that | ||
''r'' << ''R'' then, seen from the distance ''R'', the dipolar charge distribution is a ''point dipole''. | ''r'' << ''R'' then, seen from the distance ''R'', the dipolar charge distribution is a ''point dipole''. | ||
In [[physics]], one meets the following related, but different, definition: | In [[physics]], one meets the following related, but different, definition: an '''electric dipole''' of an electrostatic charge distribution ρ('''r''') is its first [[moment]], | ||
:<math> | :<math> | ||
\boldsymbol{\mu} \;\stackrel{\mathrm{def}}{=}\; \int\, \mathbf{r}\, \rho(\mathbf{r})\, \mathrm{d}\mathbf{r} | \boldsymbol{\mu} \;\stackrel{\mathrm{def}}{=}\; \int\, \mathbf{r}\, \rho(\mathbf{r})\, \mathrm{d}\mathbf{r} | ||
| Line 18: | Line 18: | ||
The first moment of this charge distribution is | The first moment of this charge distribution is | ||
:<math> | :<math> | ||
\boldsymbol{\mu} | \boldsymbol{\mu} \equiv \int\, \mathbf{r}\, \rho(\mathbf{r})\, \mathrm{d}\mathbf{r} = | ||
\int\, \mathbf{r}\, \big[q_1\delta(\mathbf{r}-\mathbf{r}_1) + q_2\delta(\mathbf{r}-\mathbf{r}_2)\big]\, \mathrm{d}\mathbf{r} | \int\, \mathbf{r}\, \big[q_1\delta(\mathbf{r}-\mathbf{r}_1) + q_2\delta(\mathbf{r}-\mathbf{r}_2)\big]\, \mathrm{d}\mathbf{r} | ||
= q_1 \mathbf{r}_1 + q_2 \mathbf{r}_2 = |q|\, (\mathbf{r}_2 - \mathbf{r}_1), | = q_1 \mathbf{r}_1 + q_2 \mathbf{r}_2 = |q|\, (\mathbf{r}_2 - \mathbf{r}_1), | ||
| Line 24: | Line 24: | ||
where we used ''q''<sub>1</sub> = −|''q''| and ''q''<sub>2</sub> = |''q''|. | where we used ''q''<sub>1</sub> = −|''q''| and ''q''<sub>2</sub> = |''q''|. | ||
This leads to a third possible definition: ''' | This leads to a third possible definition: '''an electric dipole''' is a vector pointing from a negative electric point charge to a positive electric point charge, where both charges are of the same magnitude. The size (length) of the dipole is the distance between the point charges times the magnitude of the charges. | ||
In chemistry dipole moments | In chemistry sizes of molecular dipole moments are often quoted to indicate the amount of charge separation in a [[molecule]]. Usually chemists are only interested in absolute values, a large dipole indicates a highly polar molecule (much separation of charge), whereas non-polar molecules have negligible dipoles. In the rare case that the sign of a dipole plays a role, most (organic) chemists use a convention that is opposite to the convention just discussed: they let the dipole point from a positively charged region in the molecule to a negatively charged region. | ||
==Field of point dipole== | ==Field of point dipole== | ||
Revision as of 02:06, 18 August 2009
In chemistry, an electric dipole is a charge distribution consisting of two electric point charges of opposite sign and equal magnitude.
Often it is added to this definition that the distance r between the two point charges is small—if this is the case the dipole is a point dipole. Small is a relative concept and requires further specification. In general one is interested in the electric potential field due to the dipolar charge distribution at a distance R from the midpoint of the dipole. If it holds that r << R then, seen from the distance R, the dipolar charge distribution is a point dipole.
In physics, one meets the following related, but different, definition: an electric dipole of an electrostatic charge distribution ρ(r) is its first moment,
In this definition a dipole is a vector depending on the characteristics of the charge distribution.
Combining the two definitions, one considers two point charges: q1 at position r1 and q2 at position r2. The charge distribution is written in terms of Dirac delta functions
where the charges are of opposite sign and equal magnitude. The first moment of this charge distribution is
where we used q1 = −|q| and q2 = |q|.
This leads to a third possible definition: an electric dipole is a vector pointing from a negative electric point charge to a positive electric point charge, where both charges are of the same magnitude. The size (length) of the dipole is the distance between the point charges times the magnitude of the charges.
In chemistry sizes of molecular dipole moments are often quoted to indicate the amount of charge separation in a molecule. Usually chemists are only interested in absolute values, a large dipole indicates a highly polar molecule (much separation of charge), whereas non-polar molecules have negligible dipoles. In the rare case that the sign of a dipole plays a role, most (organic) chemists use a convention that is opposite to the convention just discussed: they let the dipole point from a positively charged region in the molecule to a negatively charged region.
Field of point dipole
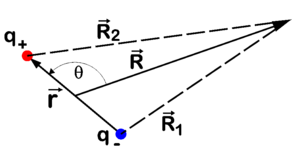
The potential field of a point dipole μ at a point R (a vector pointing from the dipole to an arbitrary in space, see the figure) is given by
where R is the length of R, the dot indicates the dot product between μ and R, and ε0 is the electric constant (vacuum permittivity).
Proof
The potential field of a point dipole can be determined fairly easily, see the figure for the symbols. In the equations we indicate vectors with bold letters, not with arrows on top as in the figure. The length of a vector is indicated with the corresponding symbol in italics. Write
Use the expansions, valid for x << 1,
Since r << R it follows that r/R << 1 and hence
The Coulomb potential at the point R is the sum of the potentials due to the two charges, which are of equal magnitude q and of opposite sign,
where we used


![{\displaystyle {\boldsymbol {\mu }}\equiv \int \,\mathbf {r} \,\rho (\mathbf {r} )\,\mathrm {d} \mathbf {r} =\int \,\mathbf {r} \,{\big [}q_{1}\delta (\mathbf {r} -\mathbf {r} _{1})+q_{2}\delta (\mathbf {r} -\mathbf {r} _{2}){\big ]}\,\mathrm {d} \mathbf {r} =q_{1}\mathbf {r} _{1}+q_{2}\mathbf {r} _{2}=|q|\,(\mathbf {r} _{2}-\mathbf {r} _{1}),}](https://wikimedia.org/api/rest_v1/media/math/render/svg/70649c77add49eb7fc6e089905417183288db7a1)

![{\displaystyle {\begin{aligned}\mathbf {R} _{1}&=\mathbf {R} +{\frac {1}{2}}\mathbf {r} &\Longrightarrow &|\mathbf {R} _{1}|^{2}={\Big (}\mathbf {R} +{\frac {1}{2}}\mathbf {r} {\Big )}\cdot {\Big (}\mathbf {R} +{\frac {1}{2}}\mathbf {r} {\Big )}=R^{2}\left[1+{\Big (}{\frac {r}{2R}}{\Big )}^{2}+{\frac {r}{R}}\cos \theta \right]\\\mathbf {R} _{2}&=\mathbf {R} -{\frac {1}{2}}\mathbf {r} &\Longrightarrow &|\mathbf {R} _{1}|^{2}={\Big (}\mathbf {R} -{\frac {1}{2}}\mathbf {r} {\Big )}\cdot {\Big (}\mathbf {R} -{\frac {1}{2}}\mathbf {r} {\Big )}=R^{2}\left[1+{\Big (}{\frac {r}{2R}}{\Big )}^{2}-{\frac {r}{R}}\cos \theta \right]\\\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1d76fc169cd5d0feeca7cbf9635092312ff364bb)


![{\displaystyle {\begin{aligned}V(\mathbf {R} )\equiv &{\frac {q_{-}}{4\pi \epsilon _{0}\,|\mathbf {R} _{1}|}}+{\frac {q_{+}}{4\pi \epsilon _{0}\,|\mathbf {R} _{2}|}}\\\approx &{\frac {q}{4\pi \epsilon _{0}\,R}}\left[-1+{\frac {r}{2R}}\cos \theta +1+{\frac {r}{2R}}\cos \theta \right]={\frac {q\;\mathbf {r} \cdot \mathbf {R} }{4\pi \epsilon _{0}\,R^{3}}}={\frac {{\boldsymbol {\mu }}\cdot \mathbf {R} }{4\pi \epsilon _{0}\,R^{3}}}\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/86ff40888871b1dd5679838765241f51abd1bb21)
