Cefalexin: Difference between revisions
imported>David E. Volk No edit summary |
imported>David E. Volk No edit summary |
||
| Line 15: | Line 15: | ||
|casnumber=15686-71-2 | |casnumber=15686-71-2 | ||
}} | }} | ||
'''Cefalexin''' (cephalexin) is one of the most widely used [[antibiotic]] medications. It is a first-generation [[cephalosporin]] type of antibiotic drug, that is sold under nearly 100 brand names. | |||
== Chemistry == | |||
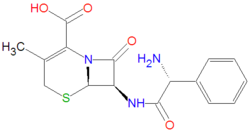
An [[IUPAC]] chemical name of cefalexin is (6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. Its chemical formula, C<sub>16</sub>H<sub>17</sub>N<sub>3</sub>O<sub>4</sub>S, gives it an average molecule mass of 347.3890 gram/mole. Its antibacterial activity is due to the core beta-[[lactam]] structure, which bind with penicillin-binding proteins within bacteria, thereby inhibiting bacterial cell wall synthesis, by an acylation reaction of the lactam with the bacterial proteins. | |||
== References == | |||
<references/> | |||
{{CZMed}} | |||
Revision as of 15:14, 13 July 2009
|
| |||||||
| cefalexin | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | beta-lactam | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Cefalexin (cephalexin) is one of the most widely used antibiotic medications. It is a first-generation cephalosporin type of antibiotic drug, that is sold under nearly 100 brand names.
Chemistry
An IUPAC chemical name of cefalexin is (6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. Its chemical formula, C16H17N3O4S, gives it an average molecule mass of 347.3890 gram/mole. Its antibacterial activity is due to the core beta-lactam structure, which bind with penicillin-binding proteins within bacteria, thereby inhibiting bacterial cell wall synthesis, by an acylation reaction of the lactam with the bacterial proteins.
References
The most up-to-date information about Cefalexin and other drugs can be found at the following sites.
- Cefalexin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Cefalexin - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Cefalexin - Detailed information from DrugBank.