Archive:New Draft of the Week: Difference between revisions
imported>Milton Beychok m (Relocated one nominee in order of vote score) |
imported>Gareth Leng |
||
| Line 24: | Line 24: | ||
| <!-- article --> {{pl|Hawaiian alphabet}} | | <!-- article --> {{pl|Hawaiian alphabet}} | ||
| <!-- score --> 3 | | <!-- score --> 3 | ||
| <!-- supporters --> [[User:Drew R. Smith|Drew R. Smith]]; [[User:Shamira Gelbman|Shamira Gelbman]];<br/>[[User:Peter Schmitt|Peter Schmitt]] | | <!-- supporters --> [[User:Drew R. Smith|Drew R. Smith]]; [[User:Shamira Gelbman|Shamira Gelbman]];<br/>[[User:Peter Schmitt|Peter Schmitt]]:[[User:Gareth Leng|Gareth Leng]] | ||
| <!-- specialist supporters --> | | <!-- specialist supporters --> | ||
| <!-- date created --> 2009-06-17 | | <!-- date created --> 2009-06-17 | ||
Revision as of 03:29, 25 June 2009
The New Draft of the Week is a chance to highlight a recently created Citizendium article that has just started down the road of becoming a Citizendium masterpiece.
It is chosen each week by vote in a manner similar to that of its sister project, the Article of the Week.
Add New Nominees Here
To add a new nominee or vote for an existing nominee, click edit for this section and follow the instructions
| Nominated article | Vote Score |
Supporters | Specialist supporters | Date created |
|---|---|---|---|---|
| 3 | Drew R. Smith; Shamira Gelbman; Peter Schmitt:Gareth Leng |
2009-06-17 | ||
| 3 | Milton Beychok, Howard C. Berkowitz; Peter Schmitt |
2009-06-11 | ||
| 3 | Milton Beychok, Howard C. Berkowitz; Peter Schmitt |
2009-06-06 | ||
| 2 | Anthony.Sebastian | 2009-06-18 | ||
| 2 | Joe Quick; Peter Schmitt |
2009-06-13 | ||
| 2 | Paul Wormer; Milton Beychok | 2009-06-18 |
If you want to see how these nominees will look on the CZ home page (if selected as a winner), scroll down a little bit.
Transclusion of the above nominees (to be done by an Administrator)
- Transclude each of the nominees in the above "Table of Nominee" as per the instructions at Template:Featured Article Candidate.
- Then add the transcluded article to the list in the next section below, using the {{Featured Article Candidate}} template.
View Current Transcluded Nominees (after they have been transcluded by an Administrator)
The next New Draft of the Week will be the article with the most votes at 1 AM UTC on Thursday, 2 July 2009. I did the honors this time. Milton Beychok 04:29, 25 June 2009 (UTC)
Text in this section is transcluded from the respective Citizendium entries and may change when these are edited.
| Nominated article | Supporters | Specialist supporters | Dates | Score
{{Featured Article Candidate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
As an oral tradition, handed down generation after generation, the true origins of the Hawaiian language are relatively unknown. The Hawaiian alphabet, ka pī‘āpā Hawai‘ i, however, does not have such an obscure past. It was originally designed in the early 1800s by American missionaries who wanted to print a Hawaiian bible. Due to the language being passed down as an oral tradition, the missionaries had to adapt the Roman alphabet to fit their needs. OriginsIn 1778, British explorer James Cook made the first reported European discovery of Hawaiʻi. In his report, he wrote the name of the islands as "Owhyhee" or "Owhyee". By July 1823, they had begun using the phrase "Hawaiian Language." The actual writing system was developed by American Protestant missionaries on January 7, 1822. The original alphabet included
ʻOkinaDue to words with different meanings being spelled alike, use of the glottal stop became necessary. As early as 1823, the missionaries made limited use of the apostrophe to represent the glottal stop, but they did not make it a letter of the alphabet. In publishing the Hawaiian bible, they used the ʻokina to distinguish koʻu ('my') from kou ('your'). It wasn’t until 1864 that the ʻokina became a recognized letter of the Hawaiian alphabet. KahakōAs early as 1821, one of the missionaries, Hiram Bingham, was using macrons in making handwritten transcriptions of Hawaiian vowels. The macron, or kahakō, was used to differentiate between short and long vowels. The macron itself never became an official letter. Instead, a second set of vowels with macrons were added to the language as separate letters. Modern AlphabetThe current official Hawaiian Alphabet consists of 18 letters: 5 normal vowels; Aa, Ee, Ii, Oo, Uu: 5 Vowels with Macrons; Āā, Ēē, Īī, Ōō, Ūū: and 8 consonants; Hh, Kk, Ll, Mm, Nn, Pp, Ww, ʻokina.Pronunciation
Diphthongs
References |
Drew R. Smith; Shamira Gelbam; Peter Schmitt | 3
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In mathematics, the continuum hypothesis is the statement that any arbitrary infinite set of real numbers has either as many elements as there are real numbers or only as many elements as there are natural numbers (i.e., there is no intermediate size). This is equivalent to the statement that there are as many real numbers as there are elements in the smallest set which is larger than the set of natural numbers. Since the set of real numbers (or the real line) is also called the continuum this can be shortly expressed as: Any set of real numbers is either countable or equivalent to the continuum. This statement was first made by Georg Cantor (1877) when he studied subsets of the real line. Cantor (who introduced sets and cardinal numbers) believed this to be true, but tried in vain to prove it. From then on it stayed, for a long time, a prominent open mathematical problem to resolve. In 1900, David Hilbert included the continuum hypothesis as the first problem, therefore also called "first Hilbert problem", in his famous lecture on 23 problems for the twentieth century. The first step towards a solution was done in 1938 by Kurt Gödel who showed that – in set theory including the axiom of choice – the (generalized) continuum hypothesis cannot be proved to be false (and thus is consistent with it). Only much later, in 1963, Paul J. Cohen showed that it cannot be proved, either. Hence the continuum hypothesis is independent of the usual (ZFC) axioms of set theory. It therefore constitutes an important, not artificially constructed, example for Gödel's Second Incompleteness Theorem. Consequently, either the continuum hypothesis or, alternatively, some contradicting assumption could be added to the axioms of set theory. But since – in contrast to the situation with the axiom of choice – there is no heuristically convincing reason to choose one of these possibilities, the "working" mathematician usually makes no use of the continuum hypotheses, and if a result depends on it, then it is explicitly mentioned. Of course, in axiomatic set theory, and especially in the theory of cardinal and ordinal numbers, the situation is different and the consequences of the various choices concerning the continuum hypothesis are extensively studied. The generalized continuum hypothesis is a much stronger statement involving the initial sequence of transfinite cardinal numbers, and is also independent of ZFC. In terms of the arithmetic of cardinal numbers (as introduced by Cantor) the continuum hypothesis reads while the generalized continuum hypothesis is Georg Cantor 1877The continuum hypothesis appears in a memoir of Cantor (dated Halle a.S., 11th July 1877, and published 1878) in which he investigates sets of real numbers. He concludes with the following remark:
Translated freely, this paragraph reads as follows:
David Hilbert 1900In his lecture on Mathematical problems, delivered before the International Congress of Mathematicians at Paris in 1900, David Hilbert states the continuum hypothesis as follows:
In the English translation which was published in 1902:
Hilbert continues this problem, now known as the "First Hilbert Problem" by describing another unproven claim of Cantor (which he thought to likely be related), namely the statement that there is a well-order of the real numbers. This property, however, turned out to be a consequence of the axiom of choice. Kurt Gödel 1947In an essay (published 1947, after his proof and before Cohen's result) Kurt Gödel argued that even if the continuum hypothesis would turn out to be independent (as he expected) this would not imply that it cannot be solved at all:
He continues with a discussion of several arguments which support his position that the continuum hypothesis is likely to be wrong. (Read more...) |
Milton Beychok; Howard C. Berkowitz; Peter Schmitt | 3
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Edward Teller was an eminent and controversial theoretical physicist. He was born as Teller Ede in Budapest (Hungary) on January 15, 1908. He died in his home on the Stanford campus (Palo Alto, California) on September 9, 2003. He had been a senior research fellow at Stanford University's Hoover Institution since 1975 when he retired as professor of the University of California, Berkeley and as associate director of the Lawrence Livermore National Laboratory. Edward Teller was one of the most controversial scientists of the 20th century because of his role as an advocate and conceptual designer of the hydrogen bomb, his outspoken defense of an unassailable nuclear arsenal, and support for President Reagan's Strategic Defensive Initiative ("Star Wars") ballistic missile defense program. During the McCarthy era he alienated many of his colleagues by his testimony in the 1954 security clearance hearings of J. Robert Oppenheimer, his former colleague and director of the Los Alamos Laboratory. YouthEdward Teller was born to Max Teller and Idona Deutsch, who both were assimilated Hungarian Jews. Edward's mother Idona was an accomplished pianist who gave up her aspirations to a concert career when she married Edward's father, who was a lawyer. As a young boy Edward experienced a short and fierce communist dictatorship under Béla Kun (March 21, 1919 – August 1, 1919); it has been suggested that his rabid aversion of communism in later life was rooted in this experience. The Hungarian communists were soon ousted by Rear Admiral Miklós Horthy who headed a fascist regime until the end of World War II. In 1918 Edward entered the famous gymnasium "Minta" ("Model"; an advanced German type of high school founded by the father of Theodore von Kármán), where he met his later wife Augusta Maria ("Mici") Harkányi, who was a sister of one of Edward's closest school friends. The Harkányis were from Jewish descent but had converted to Calvinism. After finishing the gymnasium, Edward spent a few months at the university in Budapest, but January 2, 1926 he moved to Karlsruhe in Germany to study chemical engineering. Karlsruhe was at that time the seat of one of the most outstanding technical universities of the country; especially its chemical engineering was strong because of its close cooperation with I.G. Farben, in those days world's largest chemical company. In April 1928 Edward left the field of chemical engineering and moved to Munich to study theoretical physics under Arnold Sommerfeld, a great mathematical physicist who made important contributions to the development of quantum mechanics. (Read more...) |
Milton Beychok; Howard C. Berkowitz; Peter Schmitt | 3
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
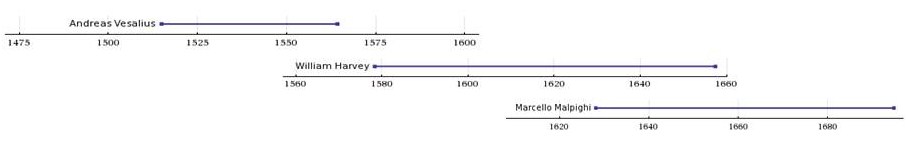
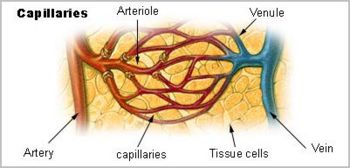
 (PD) Image: From book: William Harvey, by D'Arcy Power, 1897 William Harvey. From book of that name by D'Arcy Power, 1897 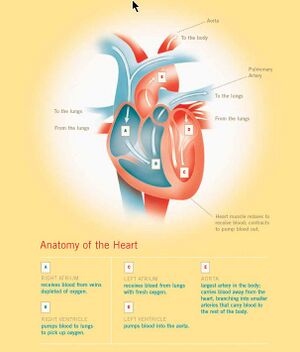 (PD) Image: Levine & Associates, Inc. for U.S National Institutes of Health, National Institute on Aging at: http://bit.ly/MnJaE Anatomy of the Human Heart. For enlarged version of this image showing more detail, click here. William Harvey (1578-1657) bestowed on humanity one of the most important advances in the history of medical science — an explanation of the core physiology of the human cardiovascular system. In part by introducing quantitative methods into anatomical and physiological investigations, Harvey discovered that the left ventricle of the heart pumps blood through the body, doing so via a system of vessels such that the blood moves in a circular path,[1] from the left side of the heart through the arteries and back to the right side of the heart through the veins, transiting from the right side of the heart to the left via blood vessels in the lung, the two sides of the heart separated by a blood-impermeable septum. He published those findings in his 1628 book, Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus (Anatomical Exercises on the Motion of the Heart and Blood in Animals), usually referred to as De Motu Cordis.[2] [3] [4] In his dissections of humans and animals, Harvey could not see vessels connecting the arteries to the veins, since, as it turns out, their minute size lies below the limits of visual acuity, even with the magnifying glass he used in his work. He had no access, and perhaps no knowledge, of the existence of microscopes, however primitive their state. Harvey could only infer that a connecting pathway existed. In 1661, a few years after Harvey died, the Italian biologist, Marcello Malpighi (1628-1694), using one of the early microscopes, discovered capillaries, tiny blood vessels not visible to the naked eye, connecting arteries to veins. In a seemingly fitting coincidence, Malpighi had entered the world the same year Harvey published De Motu Cordis. In works published little more than a century apart, 1543 to 1661, three men, Andreas Vesalius (1514-1564), William Harvey (1578-1657), and Marcello Malpighi (1628-1694), demonstrated central truths of human anatomy and physiology that had escaped Western medicine for more than a millennium following the erroneous teachings of the influential Greek physician, Galen of Pergamum (130-216 CE). It required three investigators to break the stranglehold of one.
William Harvey’s Major ContributionsAdapted from Sherwin B. Nuland (2008)[5]
Brief sketch of Williams Harvey’s lifeBorn in 1578 (April 1, at Folkstone, on the east coast of Kent, England), of Thomas and Joan Harvey, as the eldest of seven brothers and two sisters (a "week of brothers" and a "brace of sisters"), William Harvey entered the world shortly after Andreas Vesalius (1514-1564) had died, though Vesalius's reputation had not died, owing to his remarkably detailed and elegantly drawn illustrations revolutionizing the understanding of human anatomy.[7] [8] [9] For his anatomical work, William Harvey had Vesalius's giant shoulders to stand on, and ultimately he saw further. Harvey received his early education in the classics, in Canterbury, at King's School (1588-1593), there "....admonished to speak Greek or Latin even on the playground." [6] Harvey's father, a landowner and successful merchant, could afford to send Harvey to the University of Cambridge (specifically, Gonville and Caius College), which he entered at age 16 years (1593) and received his Bachelor of Arts (B.A.) degree at age 19 years (1597). Harvey developed an interest in medicine and decided to go to Italy, one of the major centers of intellectual activity in Europe at the time. He enrolled in the then renown University of Padua, studying medicine under Hieronymus Fabricius of Aquapendente, a noted anatomist in the Vesalian tradition, who had discovered the valves in the veins, a discovery which later contributed to Harvey's thinking that led to his discovery of the blood circulatory system.[10] Harvey's earlier education in the classics helped ease his learning at Padua, as lecturers spoke in Latin. Harvey received his Doctor of Medicine degree in April, 1602, at age 24 years.[11] After Padua, Harvey returned to England and developed a practice in medicine, married, and became a Fellow of the College of Physicians in London. He also secured a position as physician at St. Bartholomew’s Hospital, one of London’s great hospitals, and there and in his private practice distinguished himself as a physician. In 1615, at age 37 years, the College of Physicians elected him their Professor of Anatomy and Surgery, and gave him the honor of the Lumleian Lectureship, a lifetime remunerated position, in which he lectured on human anatomy, physiology and surgery, including performing demonstration dissections on human corpses, officially twice per week, from 1616 to 1656, the year before he died. The lecturership gave Harvey a great opportunity to organize his thinking and guide his research. His lecture notes survive as Lectures on the Whole of Anatomy as a manuscript in the British Library and in English translation.[12]
In 1618 Harvey became physician extraordinary to the king (James I), and ministered to many eminent aristocrats, including Francis Bacon, for whom he had little regard as an intellectual. After Charles I succeeded the throne, in 1625, Harvey became Charles' physician, benefitting from the King’s patronage to pursue his medical investigations. When civil strife engulfed England, Harvey, now in his 60s retired to live with a brother, pursuing his experiments until he died in 1657, having lived nearly to the age of 80 years.[14] [15] De Motu CordisTo read the full-text of De Motu Cordis in English translation, click on the "Works" tab in the banner at the beginning of this page. Equivalently, click De Motus Cordis, which brings you to same subpage of this article. A few revelatory quotes from the work:
Of course, we now know that the richer 'spirit' in arterial blood is oxygen. Before the discovery of oxygen, the alchemists of the seventeenth century recognized that air contained an essential ingredient, an 'elixir of life' — a kind of 'spirit'. We also know today that venous blood, too, has its richer 'spirit', carbon dioxide (as bicarbonate). De Generatione AnimaliumHarvey received less repute for his other great work, De Generatione Animalium — On the Generation of Animals — a contribution to embryology.... References cited and notes
|
Anthony Sebastian | 2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Heat is a form of energy that is transferred between two bodies that are in thermal contact and have different temperatures. For instance, the bodies may be two compartments of a vessel separated by a heat-conducting wall and containing fluids of different temperatures on either side of the wall. Or one body may consist of hot radiating gas and the other may be a kettle with cold water, as shown in the picture. Heat flows spontaneously from the higher-temperature to the lower-temperature body. The effect of this transfer of energy usually, but not always, is an increase in the temperature of the colder body and a decrease in the temperature of the hotter body. Change of aggregation stateA vessel containing a fluid may lose or gain energy without a change in temperature when the fluid changes from one aggregation state to another. For instance, a gas condensing to a liquid does this at a certain fixed temperature (the boiling point of the liquid) and releases condensation energy. When a vessel, containing a condensing gas, loses heat to a colder body, then, as long as there is still vapor left in it, its temperature remains constant at the boiling point of the liquid, even while it is losing heat to the colder body. In a similar way, when the colder body is a vessel containing a melting solid, its temperature will remain constant while it is receiving heat from a hotter body, as long as not all solid has been molten. Only after all of the solid has been molten and the heat transport continues, the temperature of the colder body (then containing only liquid) will rise. For example, the temperature of the tap water in the kettle shown in the figure will rise quickly to the boiling point of water (100 °C). Then, when the flame is not switched off, the temperature inside the kettle remains constant at 100 °C for quite some time, even though heat keeps on flowing from flame to kettle. When all liquid water has evaporated—when the kettle has boiled dry—the temperature of the kettle will quickly rise again until it obtains the temperature of the burning gas, then the heat flow will finally stop. (Most likely, though, the handle and maybe the metal of the kettle, too, will have melted before that). UnitsAt present the unit for the amount of heat is the same as for any form of energy. Before the equivalence of mechanical work and heat was clearly recognized, two units were used. The calorie was the amount of heat necessary to raise the temperature of one gram of water from 14.5 to 15.5 °C and the unit of mechanical work was basically defined by force times path length (in the old cgs system of units this is erg). Now there is one unit for all forms of energy, including heat. In the International System of Units (SI) it is the joule, but the British Thermal Unit and calorie are still occasionally used. The unit for the rate of heat transfer is the watt (J/s). Equivalence of heat and workAlthough heat and work are forms of energy that both obey the law of conservation of energy, they are not completely equivalent. Work can be completely converted into heat, but the converse is not true. When converting heat into work, part of the heat is not—and cannot be—converted to work, but flows to the body of lower temperature that is out of necessity present to generate a heat flow. Heat and temperatureThe important distinction between heat and temperature (heat being a form of energy and temperature a measure of the amount of that energy present in a body) was clarified by Count Rumford, James Prescott Joule, Julius Robert Mayer, Rudolf Clausius, and others during the late 18th and 19th centuries. Also it became clear by the work of these men that heat is not an invisible and weightless fluid, named caloric, as was thought by many 18th century scientists, but a form of motion. The molecules of the hotter body are (on the average) in more rapid motion than those of the colder body. The first law of thermodynamics, discovered around the middle of the 19th century, states that the (flow of) heat is a transfer of part of the internal energy of the bodies. In the case of ideal gases, internal energy consists only of kinetic energy and it is indeed only this motional energy that is transferred when heat is exchanged between two containers with ideal gases. In the case of non-ideal gases, liquids and solids, internal energy also contains the averaged inter-particle potential energy (attraction and repulsion between molecules), which depends on temperature. So, for non-ideal gases, liquids and solids, also potential energy is transferred when heat transfer occurs. Forms of heatThe actual transport of heat may proceed by electromagnetic radiation (as an example one may think of an electric heater where usually heat is transferred to its surroundings by infrared radiation, or of a microwave oven where heat is given off to food by microwaves), conduction (for instance through a metal wall; metals conduct heat by the aid of their almost free electrons), and convection (for instance by air flow or water circulation). EntropyIf two systems, 1 (cold) and 2 (hot), are isolated from the rest of the universe (i.e., no other heat flows than from 2 to 1 and no work is performed on the two systems) then the entropy Stot = S1 + S2 of the total system 1 + 2 increases upon the spontaneous flow of heat. This is in accordance with the second law of thermodynamics that states that spontaneous thermodynamic processes are associated with entropy increase. In general, the entropy S of a system at absolute temperature T increases with when it receives an amount of heat Q > 0. Entropy is an additive (size-extensive) property. The hotter system 2 loses an amount of heat to the colder system 1. In absolute value the exchanged amounts of heat are the same by the law of conservation of energy (no energy escapes to the rest of the universe), hence Here it is assumed that the amount of heat Q is so small that the temperatures of the two systems are constant. One can achieve this by considering a small time interval of heat exchange and/or very large systems. Remark: the expression ΔS = Q/T is only strictly valid for a reversible (also known as quasistatic) flow of energy. It is possible[1] to define: It is assumed that ΔSint is much smaller than ΔSext, so that it can be neglected. Semantic caveatsIt is strictly speaking not correct to say that a hot object "possesses much heat"—it is correct to say, however, that it possesses high internal energy. The word "heat" is reserved to describe the process of transfer of energy from a high temperature object to a lower temperature one (in short called "heating of the cold object"). The reason that the word "heat" is to be avoided for the internal energy of an object is that the latter can have been acquired either by heating or by work done on it (or by both). When we measure internal energy, there is no way of deciding how the object acquired it—by work or by heat. In the same way as one does not say that a hot object "possesses much work", one does not say that it "possesses much heat". Yet, terms as "heat reservoir" (a system of temperature higher than its environment that for all practical purposes is infinite) and "heat content" (a synonym for enthalpy) are commonly used and are incorrect by the same reasoning. The molecules of a hot body are in agitated motion and, as said, it cannot be measured how they became agitated, by work or by heat. Often, especially outside physics, the random molecular motion is referred to as "thermal energy". In classical (phenomenological) thermodynamics this is an intuitive, but undefined, concept. In statistical thermodynamics, thermal energy could be defined (but rarely ever is) as the average kinetic energy of the molecules constituting the body. Kinetic and potential energy of molecules are concepts that are foreign to classical thermodynamics, which predates the general acceptance of the existence of molecules. Quotation
Reference (Read more...)
|
Joe Quick; Peter Schmitt | 2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The Air Quality Index (AQI), also known as the Air Pollution Index (API), Pollutant Standard Index (PSI) or Air Quality Health Index (AQHI), is a number used by government agencies to characterize the quality of the ambient air at a given location. As the AQI increases, the severity of probable adverse health effects increases as does the percentage of the population expected to be affected by the adverse health effects. To compute the AQI requires an air pollutant concentration to be obtained from an air quality monitoring station. The method used to convert from air pollutant concentrations to AQIs varies for each air pollutant, and is different in different countries. In many countries, air quality index values are divided into ranges, and each range is assigned a descriptor (i.e., a very few words describing the air quality or the health effects of the range) and often a color code as well. A government agency might also encourage members of the public to avoid strenuous activities, use public transportation rather than personal automobiles and work from home when AQI levels are high. Many countries monitor ground-level ozone, particulate matter (PM10), sulfur dioxide (S02), carbon monoxide (CO) and nitrogen dioxide (NO2) and calculate air quality indices for these pollutants. Most other air contaminants do not have an associated AQI. Air Quality Indices by country
CanadaEnvironment Canada, the national environmental protection agency of Canada, uses Air Quality Health Index (AQHI) categories ranging from 1 to 10+ and each category has an assigned color code (see adjacent table) that enables members of the general public to easily identify their health risks as indicated in published air quality forecasts.[1] As shown in the adjacent table:
As of 2009, many of the Canadian provinces, if not all, have adopted the AQHI categories implemented by Environment Canada. China
China's Ministry of Environmental Protection (MEP)[2][4] is responsible for monitoring the level of air pollution in China. As of August 2008, MEP monitors daily pollution level in its major cities and develops an Air Pollution Index (API) level that is based on the ambient air concentrations sulfur dioxide, nitrogen dioxide, particulate matter (PM10), carbon monoxide, and ozone as measured at monitoring stations in each of those major cities.[2][4] The adjacent table presents China's national API scale, which is not color coded and uses a scale 0 to more than 300, divided into five ranges of air quality categorized as excellent, good, slightly polluted, heavily polluted and hazardous. API MechanicsAn individual score is assigned to the level of each pollutant and the final API is the highest of those 5 scores. The pollutant concentrations are obtained quite differently. Sulfur dioxide, nitrogen dioxide and PM10 concentrations are obtained as daily averages. Carbon monoxide and ozone are more harmful and are obtained as an hourly averages. The final API value is calculated as a daily average.[2][4] The scale for each pollutant is non-linear, as is the final daily API value. Thus, an API value of 100 does not mean it is twice the pollution of API at 50, nor does it mean it is twice as harmful. Beijing's APIChina's capitol city, Beijing, has its own API scale, which was developed by the Beijing Municipal Environmental Protection Bureau.[5] As can be seen in the adjacent table, the API scale used by Beijing differs quite significantly from China's national scale in that: • The Beijing scale ranges from 0 to 500 (rather than 0 to 300 as in the national scale) Hong KongThe Hong Kong Environmental Protection Department (Hong Kong EPD) has developed a color coded Air Pollution Index (API) based upon the measured concentrations of ambient particulate matter (PM10), sulfur dioxide, carbon monoxide, ozone and nitrogen dioxide over a 24-hour period. Hong Kong's color coded Air Pollution Index (API) scale ranges from 0 to 500 corresponding to adverse health effects that range from low to severe as shown in the adjacent chart:[3]
Although Hong Kong is now part of China, it can be seen that Hong Kong's API scale differs from both China's scale and Beijing's scale.
MalaysiaThe air quality in Malaysia is described in terms of an Air Pollutant Index (API). The API is an indicator of air quality and was developed based on scientific assessment to indicate in an easily understood manner, the presence of pollutants and its impact on health. The API system of Malaysia closely follows the similar system developed by the U.S. Environmental Protection Agency (U.S. EPA). As shown in the adjacent table, Malaysia does not color code their air quality categories. Monitoring stations measure the concentration of five major pollutants in the ambient air: PM10, sulfur dioxide, nitrogen dioxide, carbon monoxide and ozone. These concentrations are measured continuously on an hourly basis. The hourly value is then averaged over a 24-hour period for PM110 and sulfur dioxide and an 8-hour period for carbon monoxide. The ozone and nitrogen dioxide are read hourly. An hourly index is then calculated for each pollutant. The highest hourly index value is then taken as the API for the hour. When the API exceeds 500, a state of emergency is declared in the reporting area. Usually, this means that non-essential government services are suspended, and all ports in the affected area closed. There may also be a prohibition on private sector commercial and industrial activities in the reporting area excluding the food sector.
MexicoThe air quality in Mexico is described and reported hourly in terms of a color coded Metropolitan Index of Air Quality (IMECA), developed by the Ministry of the Environment for the Government of the Federal District. The IMECA is calculated from the results of real-time monitoring of the ambient concentrations of ozone, sulfur dioxide, nitrogen dioxide, carbon monoxide and particulate matter (PM10). The IMECA was developed specifically for the Federal District of Mexico which only encompasses Mexico City and its surrounding suburbs and adjacent municipalities. The real-time monitoring of the ambient atmosphere is performed by the Sistema de Monitoreo Atmosférico de la Ciudad de México (SIMAT or System of Atmospheric Monitoring for Mexico City). SIMAT's real-time monitoring includes monitoring of the ultra-violet (UV) radiation from the sun and the results are also described and reported hourly as IUVs (Índice de Radiación Ultravioleta) in a manner that is similar to the reporting of the IMECAs.[8]
SingaporeSingapore's National Environment Agency (NEA) in the Ministry of the Environment and Water Resources (MEWR) has the responsibility for the real-time monitoring of the concentrations of sulfur dioxide, nitrogen dioxide, carbon monoxide, ozone and PM10 in the ambient air of Singapore. The real-time monitoring of the ambient air quality is done by a telemetric network of air quality monitoring stations strategically located in different parts of Singapore. The NEA uses the real-time monitoring data to obtain and report 24-hour Pollution Standard Index (PSI) levels along with their corresponding air quality categories as shown in the adjacent table and which does not use color coding.[9] The NEA states that the PSI scale developed for use in Singapore is very similar to the scale developed and used by the U.S. Environmental Protection Agency. The NEA also further states that the National Ambient Air Quality Standards (NAAQS) developed by the U.S. Environmental Protection Agency are used to assess Singapore's air quality. Although the adjacent table indicates that the NEA categorizes a 24-hour PSI level that is higher than 300 as being hazardous, the NEA also considers a 24-hour PSI level higher than 400 to be life-threatening to ill and elderly persons.[10]
United KingdomAEA Technology, a British environmental consulting company, issues air quality forecasts for the United Kingdom (UK) on behalf of the Department for Environment, Food and Rural Affairs (Defra).[11] The scale used in the United Kingdom is an Air Pollution Index (API) with levels ranging from 1 to 10 as shown in the attached table and it is color coded. The scale was thoroughly studied and approved by the United Kingdom's government advisory body, namely the "Committee on Medical Effects of Air Pollution Episodes" (COMEAP).[11] The scale is based on continuous monitoring, in locations throughout the United Kingdom, of the ambient air for the concentrations of the major air pollutants, namely sulfur dioxide, nitrogen dioxide, ozone, carbon monoxide and PM10. The forecasts issued by AEA Technology are based on the prediction of air pollution index for the worst-case of the five pollutants. As shown in the adjacent table, the health effect of each API range is referred to as its banding rather than as its category. The health effect bandings for the API ranges are low, moderate, high and very high.
United StatesThe Air Quality Index (AQI) ranges used by the U.S. Environmental Protection Agency (U.S. EPA) and their corresponding health effect categories and color codes are provided in the adjacent table. The U.S. EPA's AQI is also known as the Pollution Standards Index (PSI). If multiple pollutants are measured at a monitoring site, then the largest or "dominant" AQI value is reported for the location. The U.S. EPA has developed conversion calculators, available online,[13][14] for the conversion of AQI values to concentration values and for the reverse conversion of concentrations to AQI values. A national map of the United States of America containing daily AQI forecasts across the nation, developed jointly by the U.S. EPA and NOAA is also available online.[15] The U.S. Clean Air Act requires the U.S. EPA to review its National Ambient Air Quality Standards[16] every five years to reflect evolving health effects information. The Air Quality Index is adjusted periodically to reflect these changes. Air pollutant concentration measurement unitsIn the United States, the concentrations of the air pollutants involved in the AQI are usually expressed as:
References
|
Paul Wormer; Milton Beychok | 2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Current Winner (to be selected and implemented by an Administrator)
To change, click edit and follow the instructions, or see documentation at {{Featured Article}}.
| This article may be deleted soon. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Now and Zen is an album by the former Led Zeppelin singer Robert Plant, released in 1988 via the record label Es Paranza. The album generated two Mainstream Rock Tracks|mainstream rock hits, 'Heaven Knows' (Number 1 for six weeks) and 'Tall Cool One' (Number 1 for four weeks), and earned Plant his first solo multi-platinum honour with RIAA.[1] OverviewWith a new backing band and time to rethink the direction of his career, Plant returned in late 1987 with more of the material that had historically defined him in Led Zeppelin. Although Plant persisted in utilising computerised audio technology, in a comparable fashion to his anteceding solo issues, for this release Plant re-integrated blues-rock that had all but been relinquished on his 1985 release Shaken 'n' Stirred.[2] Plant, who often uses mysterious and mystical lyrics, composes some of his most coherent songs, and the manner in which the writing complements the melodic arrangements are partially responsible for the commercial success of Now and Zen. A prominent guitar and an exotic aural texture to the recordings also marked another transformation in Plant's sound, who now added Middle Eastern colouration in compositions like 'Heaven Knows'. This is a musical direction that he would eventually re-engage with in the mid-1990s with the Jimmy Page and Robert Plant project. This album is also notable in that it marks his first collaboration with keyboardist Phil Johnstone, who would continue to play and write with Plant on subsequent albums, and song-writer producer Dave Barrett. Plant's lifelong loyalty to his favourite Association football|football team Wolverhampton Wanderers (The Wolves) is expressed in the form of wolf motifs on the front cover. The working title for this recording project was in fact Wolves. In another symbolic return to his past, Plant's feather from Led Zeppelin IV in encapsulated in a crystal, next to the wolf motifs. The charting singles 'Heaven Knows' and 'Tall Cool One' features Led Zeppelin guitarist Jimmy Page (On the liner notes, Page's participation on the recordings were signified with a ZoSo symbol)[3], underpinning a riff similar to the Yardbirds-era standard 'The Train Kept a-Rollinˈ'. In retort to the Beastie Boys' unauthorised sampling of Led Zeppelin songs on their 1986 album Licensed to Ill, Plant also sampled Led Zeppelin tracks ('Whole Lotta Love', 'Black Dog', 'The Ocean (song)|The Ocean', and 'Custard Pie') on 'Tall Cool One', furthermore singing lyrical refrains from 'When the Levee Breaks (Led Zeppelin song)|When the Levee Breaks'.[4] Plant reflects with 'White, Clean and Neat', a song evoking teen life in the mid-1950s, when the arrival of rock 'n' roll divided families and whole generations. 'Walking Towards Paradise' was initially a bonus track obtainable only on the CD version of the album. Rhino Entertainment eventually issued a remastered edition of the album, with additional tracks, on 3 April 2007. Plant performed 'Heaven Knows', 'Tall Cool One', and 'Ship of Fools' at the Atlantic Records 40th Anniversary concert in 1988. 'Ship of Fools' was also used on the final two-hour episode of Miami Vice entitled 'Freefall'. In an interview he gave to Uncut (magazine)|Uncut magazine in 2005, Plant commented:
Track list
Chart positionsAlbum
Singles
CertificationsAlbum
Credits
Notes
Previous Winners
Rules and ProcedureRules
NominationSee above section "Add New Nominees Here". Voting
Ranking
Updating
AdministratorsThe Administrators of this program are the same as the admins for CZ:Article of the Week. ReferencesSee Also
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||