Antibiotic: Difference between revisions
imported>David E. Volk m (→Penicillin-like, beta-lactam drugs: Hetacillin) |
imported>David E. Volk (→to be catagorized yet: Cephalosporin list) |
||
| Line 23: | Line 23: | ||
* [[Penicillin V]] | * [[Penicillin V]] | ||
* [[Piperacillin]] | * [[Piperacillin]] | ||
== Cephalosporins == | |||
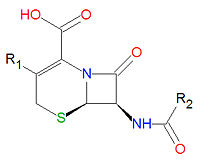
[[Image:Cephalosporin base.jpg|right|thumb|200px|{{#ifexist:Template:Cephalosporin base.jpg/credit|{{Cephalosporin base.jpg/credit}}<br/>|}}Base structure of all cephalosporins.]] | |||
'''Cephalosporins''' are a class of [[antibiotic]] compounds sharing a common base structure, 7-aminocephalosporanic acid (7-ACA), that was derived from the first cephalosporin discovered, [[cephalosporin C]]. [[Penicillin]]s are very similar, although they contain a five-membered ring in place of the six-membered ring present in the cephalosporin. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of a [[lactam|beta-lactam]], which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins are often made semisynthetically. Cephalosporins and the very closely related[[cephamycin]]s are collectively referred to as [[cephems]]. | |||
== Nomenclature == | |||
Because the original cephalosporins used the "ceph" form of the spelling and were often trademarked, the [[International Nonproprietary Name]]s (INN) suggested by the [[World Health Organization]] use the "cef" spelling for the generic drug name of all cephalosporins. | |||
== First generation cephalosporins == | |||
* [[Cefacetrile]] | |||
* [[Cefadroxil]] | |||
* [[Cefalexin]] | |||
* [[Cefaloglycin]] | |||
* [[Cefalonium]] | |||
* [[Cefaloridine]] | |||
* [[Cefalotin]] | |||
* [[Cefapirin]] | |||
* [[Cefatrizine]] | |||
* [[Cefazaflur]] | |||
* [[Cefazedone]] | |||
* [[Cefazolin]] | |||
* [[Cefradine]] | |||
* [[Cefroxadine]] | |||
* [[Ceftezole]] | |||
== Second generation cephalosporins == | |||
In general, second generation cephalosporins have a broader spectrum of activity against [[Gram-negative]] bacteria. | |||
* [[Cefaclor]] | |||
* [[Cefonicid]] | |||
* [[Cefprozil]] | |||
* [[Cefuroxime]] | |||
* [[Cefuzonam]] | |||
* [[Cefmetazole]] | |||
* [[Cefotetan]] | |||
* [[Cefoxitin]] | |||
== Third generation cephalosporins == | |||
* [[Cefcapene]] | |||
* [[Cefdaloxime]] | |||
* [[Cefdinir]] | |||
* [[Cefditoren]] | |||
* [[Cefetamet]] | |||
* [[Cefixime]] | |||
* [[Cefmenoxime]] | |||
* [[Cefodizime]] | |||
* [[Cefotaxime]] | |||
* [[Cefpimizole]] | |||
* [[Cefpodoxime]] | |||
* [[Cefteram]] | |||
* [[Ceftibuten]] | |||
* [[Ceftiofur]] | |||
* [[Ceftiolene]] | |||
* [[Ceftizoxime]] | |||
* [[Ceftriaxone]] | |||
* [[Cefoperazone]] | |||
* [[Ceftazidime]] | |||
==to be catagorized yet == | ==to be catagorized yet == | ||
Revision as of 13:02, 11 July 2008
Antibiotics are defined as "substances that reduce the growth or reproduction of bacteria."[1] They interfere with the life cycle of bacteria in a number of different ways. Some antibiotics, like penicillin, interfere with cell wall synthesis, while other are reverse transcriptase inhibitors that interefere with the production of viral RNA and DNA. Other antibiotics are nucleoside analogs that get incorporated into the viral RNA or DNA and act a chain terminators.
Misuse
One study on respiratory tract infections found "physicians were more likely to prescribe antibiotics to patients who they believed expected them, although they correctly identified only about 1 in 4 of those patients".[2] Multifactorial interventions aimed at both physicians and patients can reduce inappropriate prescribing of antibiotics. [3] Delaying antibiotics for 48 hours while observing for spontaneous resolution of respiratory tract infections may reduce antibiotic usage; however, this strategy may reduce patient satisfaction.[4]
List of antibiotics for systemic use
Penicillin-like, beta-lactam drugs
- Amoxicillin
- Ampicillin
- Azlocillin
- Carbenicillin
- Cloxacillin
- Dicloxacillin
- Flucloxacillin
- Hetacillin
- Oxacillin
- Mezlocillin
- Penicillin G
- Penicillin V
- Piperacillin
Cephalosporins
Cephalosporins are a class of antibiotic compounds sharing a common base structure, 7-aminocephalosporanic acid (7-ACA), that was derived from the first cephalosporin discovered, cephalosporin C. Penicillins are very similar, although they contain a five-membered ring in place of the six-membered ring present in the cephalosporin. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of a beta-lactam, which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins are often made semisynthetically. Cephalosporins and the very closely relatedcephamycins are collectively referred to as cephems.
Nomenclature
Because the original cephalosporins used the "ceph" form of the spelling and were often trademarked, the International Nonproprietary Names (INN) suggested by the World Health Organization use the "cef" spelling for the generic drug name of all cephalosporins.
First generation cephalosporins
- Cefacetrile
- Cefadroxil
- Cefalexin
- Cefaloglycin
- Cefalonium
- Cefaloridine
- Cefalotin
- Cefapirin
- Cefatrizine
- Cefazaflur
- Cefazedone
- Cefazolin
- Cefradine
- Cefroxadine
- Ceftezole
Second generation cephalosporins
In general, second generation cephalosporins have a broader spectrum of activity against Gram-negative bacteria.
Third generation cephalosporins
- Cefcapene
- Cefdaloxime
- Cefdinir
- Cefditoren
- Cefetamet
- Cefixime
- Cefmenoxime
- Cefodizime
- Cefotaxime
- Cefpimizole
- Cefpodoxime
- Cefteram
- Ceftibuten
- Ceftiofur
- Ceftiolene
- Ceftizoxime
- Ceftriaxone
- Cefoperazone
- Ceftazidime
to be catagorized yet
- Amikacin
- Azithromycin
- Aztreonam
- Cefaclor
- Cefadroxil
- Cefdinir
- Cefditoren Pivoxil
- Cefixime
- Cefmenoxime
- Cefmetazole
- Ceforanide
- Cefotaxime
- Cefotiam
- Cefpiramide
- Cefprozil
- Ceftazidime
- Ceftriaxone
- Cefuroxime
- Cephalexin
- Chloramphenicol
- Cinoxacin
- Ciprofloxacin
- Clarithromycin
- Clindamycin
- Clomocycline
- Colistin
- Demeclocycline
- Dirithromycin
- Doxycycline
- Enoxacin
- Ertapenem
- Erythromycin
- Fosfomycin
- Gatifloxacin
- Gemifloxacin
- Gentamicin
- Grepafloxacin
- Hetacillin
- Kanamycin
- Levofloxacin
- Linezolid
- Lomefloxacin
- Loracarbef
- Lymecycline
- Meropenem
- Metronidazole
- Minocycline
- Moxifloxacin
- Nalidixic Acid
- Netilmicin
- Neomycin
- Nitrofurantoin
- Norfloxacin
- Ofloxacin
- Oxytetracycline
- Pefloxacin
- Polymyxin B Sulfate
- Procaine
- Roxithromycin
- Sparfloxacin
- Spectinomycin
- Streptomycin
- Sulfadiazine
- Sulfamethizole
- Sulfamethoxazole
- Sulfanilamide
- Sulfapyridine
- Telithromycin
- Tetracycline
- Tinidazole
- Trimethoprim
- Tobramycin
- Trovafloxacin
- Vancomycin
References
- ↑ National Library of Medicine. Antiobiotics. Retrieved on 2007-11-15.
- ↑ Ong S, Nakase J, Moran GJ, Karras DJ, Kuehnert MJ, Talan DA (2007). "Antibiotic use for emergency department patients with upper respiratory infections: prescribing practices, patient expectations, and patient satisfaction". Annals of emergency medicine 50 (3): 213-20. DOI:10.1016/j.annemergmed.2007.03.026. PMID 17467120. Research Blogging.
- ↑ Metlay JP, Camargo CA, MacKenzie T, et al (2007). "Cluster-randomized trial to improve antibiotic use for adults with acute respiratory infections treated in emergency departments". Annals of emergency medicine 50 (3): 221-30. DOI:10.1016/j.annemergmed.2007.03.022. PMID 17509729. Research Blogging.
- ↑ Spurling G, Del Mar C, Dooley L, Foxlee R (2007). "Delayed antibiotics for respiratory infections". Cochrane database of systematic reviews (Online) (3): CD004417. DOI:10.1002/14651858.CD004417.pub3. PMID 17636757. Research Blogging.
- Pages using PMID magic links
- CZ Live
- Health Sciences Workgroup
- Chemistry Workgroup
- Infectious Disease Subgroup
- Pharmacology Subgroup
- Biochemistry Subgroup
- Articles written in American English
- Advanced Articles written in American English
- All Content
- Health Sciences Content
- Chemistry Content
- Infectious Disease tag
- Pharmacology tag