Phosphorus
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Phosphorus is a chemical element, with atomic number Z = 15, that is present in all living organisms
in the form of organophosphates and as calcium phosphates such as hydroxyapetite (Ca10(PO4)6(OH)2) and fluoroapatite (Ca10(PO4)6F2) found in teeth and bones. Many cell signaling cascades in living organisms operate by a series of phosphorylation events in which a phosphate group (PO4)2− is either added to a protein by a kinase or removed from a protein by a phosphorylase.
Unlike other elements in group VA of the periodic table, phosphorus is never found as a pure element in nature, but only in combination with other elements. Both red phosphorus and tetraphosphorus trisulfide are used in common matches because they are easily ignited by heat. However, the agricultural industry is the largest user of phosphorus in the form of fertilizers. The radioactive isotope 32P is used to radiolabel compounds for scientific studies. Phosphorus and arsenic share many chemical properties.
Production of elemental phosphorus
Calcium phosphate (phosphate rock), mostly mined in Florida and North Africa, can be heated to 1200-1500 Celcius with sand, which is mostly SiO2, and coke (impure carbon) to produce vaporized tetraphosphorus, P4, (mp. 44.2 C) which is subsequently condensed into a white power under water to prevent oxidation. Even under water, white phosphorus is slowly converted to the more stable red phosphorus allotrope (mp. 597C). Both the white and red allotropes of phosphoruus are insoluble in water.
Fertilizers
Due to the essential nature of phosphorus to living organisms, the low solubility of natural phosphorus-containing compounds, and the slow natural cycle of phosphorous, the agricultural industry is heavily reliant on fertilizers which contain phosphate, mostly in the form of superphosphate of lime. Superphosphate of lime is a mixture of two phosphate salts, calcium dihyrogen phosphate (Ca(H2PO4)2) and calcium sulfate dihydrate CaSO4•2H2O produced by the reaction of sulfuric acid and water with calcium phosphate.
Allotropes of phosphorus
Both phosphorus and arsenic have many allotropes, but only the white and red forms predominate.
- White phosphorus and yellow arsenic both have four atoms arranged in a tetrahedral structure in which each atom is bound to the other three atoms by a single bond. This form of the elements are the least stable, most reactive, more volatile, less dense, and more toxic than the other allotropes. The toxicity of white phosphorus lead to its discontinued use in matches.
- Red phosphorus: here one of the bonds in P4 described above has been broken, and one additional bond is formed with a neighboring tetrahedron.
- Black phosphorus is made of even larger aggregates. P2, which contains a triple bond and is analogous to N2, is stable only at high temperatures.
Phosphine, diphosphine and phosphonium salts
Phosphine (PH3) and arsine (AsH3) are structural analogs with ammonia (NH3) and form pyramidal structures with the phosphorus or arsenic atom in the center bound to three hydrogen atoms and one lone electron pair. Both are colorless, ill-smelling, toxic compounds. Phosphine is produced in a manner similar to the production of ammonia. Hydrolysis of calcium phosphide, Ca3P2, or calcium nitride, Ca3N2 produce phosphine or ammonia, respectively. Unlike ammonia, phosphine is unstable and it reacts instantly with air giving off phosphoric acid clouds. Arsenic is even less stable. Although phosphine is less basic than ammonia, it can form a few some phosphonium salts (PH4I), analogs of ammonium salts, but these salts immediately decompose in water and do not yield PH4+ ions. Diphosphine (P2H4 or H2P-PH2) is an analog of hydrazine (N2H4) that is a colorless liquid which spontaneously ignites in air and can disproportionate into phosphine and complex hydrides.
Phosphorus halides
The trihalides PF3, PCl3, PBr3 and PI3 and the pentahalides PF5, PCl5 and PBr5 are all known and mixed halides can also be formed. The trihalides can be formed simply by mixing the appropriate stoichiometric amounts of phosphorus and a halide. For safety reasons, however, PF3 is typically made by reacting PCl3 with AsF5 and fractional distillation because the direct reaction of phosphorus with fluorine can be explosive. The pentahalides, PX5, are synthesized by reacting excess halide with either elemental phosphorus or with the corresponding trihalide. Mixed phosphorus halides are unstable and decompose to form simple halides. Thus 5PF3Br2 decomposes into 3PF5 and 2PBr5.
Phosphorus oxides (acid anhydrides)
Phosphorus(III)oxide, P4O6 (also called tetraphosphorus hexoxide) and phosphorus(IV) oxide, P4O10 (or tetraphosphorus decoxide) are acid anhydrides of phosphorus oxyacids and hence readily react with water. P4O10 is a particularly good dehydrating agent that can even remove water from nitric acid, HNO3. The structure of P4O6 is like that of P4 with an oxygen atom inserted between each of the P-P bonds. The structure of P4O10 is like that of P4O6 with the addition of one oxygen bond to each phosphorus atom via a double bond and protruding away from the tetrahedral structure.
Phosphorus oxyacids
Phosphorous oxyacids can have acidic protons bound to oxygen atoms and nonacidic protons which are bonded directly to the phosphorus atom. Although many oxyacids of phosphorus are formed, only six are important (see table), and three of them, hypophosphorous acid, phosphorous acid and phosphoric acid are particularly important ones.
| Oxidation State | Formula | Name | Acidic Protons | Compounds |
|---|---|---|---|---|
| +1 | H3PO2 | hypophosphorous acid | 1 | acid, salts |
| +3 | H3PO3 | (ortho)phosphorous acid | 2 | acid, salts |
| +5 | (HPO3)n | metaphosphoric acids | n | salts (n=3,4) |
| +5 | H5P3O10 | triphosphoric acid | 3 | salts |
| +5 | H4P2O7 | pyrophosphoric acid | 4 | acid, salts |
| +5 | H3PO4 | phosphoric acid | 3 | acid, salts |
Widely used phosphorous compounds
| Phosphorous compound | Use |
|---|---|
| Ca(H2PO4)2•H2O | Baking powder & fertilizers |
| CaHPO4•2H2O | Animal food additive, toothpowder |
| H3PO4 | Manufacture of phosphate fertilizers |
| PCl3 | Manufacture of POCl3 and pesticides |
| POCl3 | plasticizer Manufacturing |
| P4S10 | Manufacturing of additives and pesticides |
| Na5P3O10 | Detergents |
Isotopes of phosphorus
Although twenty three isotopes of phosphorus are known (all posibilities from 24P up to 46P), only 31P, with spin 1/2, is stable and is therefore present at 100% abundance. The half-integer spin and high abundance of 31P make it useful for nuclear magnetic resonance studies of biomolecules, particularly DNA.
Two radioactive isotopes of phosphorous have half-lives which make them useful for scientific experiments. 32P has a half-life of 14.262 days and 33P has a half-life of 25.34 days. Biomolecules can be "tagged" with a radio isotope to allowed for the study of very dilute samples.
Biological membranes and phospholipids
All cells must have a membrane that distinguishes it from the cell's surrounding. Biological membranes are made from a phospholipid matrix and proteins, typically in the form of a bilayer. Phospholipids are derived from glycerol, such that two of the glycerol hydroxyl (OH) protons have been replaced with fatty acids as an ester, and the third hydroxyl proton has been replaced with phosphate bonded to another alcohol.
History
Phosphorus was discovered by the Hamburg alchemist Hennig Brand in 1669. He made it out of human urine. He let urine stand for days until it gave off a terrible smell. Then he boiled it down to a paste, heated this paste to a high temperature, and led the vapors through water where he hoped they would condense to gold. Instead, he obtained a white, waxy substance that glowed in the dark. Brand had discovered phosphorus, the first element discovered since antiquity. The word phosphorus comes from the Greek (φως = light, φορέω = carry) and means light carrier. We now know that Brand produced ammonium sodium hydrogenphosphate (NH4)NaHPO4.
Brand at first tried to keep the method secret, but later sold the recipe for 200 thaler to somebody from Dresden, Krafft, who could now make it as well, and toured much of Europe with it, including England, where he met with Robert Boyle. The secret that it was made from urine leaked out and first Johann Kunckel (1630-1703) in Sweden (1678) and later Boyle in London (1680) also managed to make phosphorus. Boyle states that Krafft gave him no no information as to the preparation of phosphorus other than that it was derived from "somewhat that belonged to the body of man". This gave Boyle a valuable clue, however, so that he, too, managed to make phosphorus. Later he improved Brand's process by using sand in the reaction (still using urine as base material),
- 4NaPO3 + 2SiO2 + 10C → 2Na2SiO3 + 10CO + P4
Robert Boyle was the first to use phosphorus to ignite sulphur-tipped wooden splints, forerunners of our modern matches, in 1680.
In 1769 Johan Gottlieb Gahn and Carl Wilhelm Scheele showed that calcium phosphate (Ca3(PO4)2) is found in bones and they obtained phosphorus from bone ash. Antoine Lavoisier recognized phosphorus as an element in 1777. Bone ash was the major source of phosphorus until the 1840s. Phosphate rock, a mineral containing calcium phosphate, was first used in 1850 and following the introduction of the electric arc furnace in 1890 this became the only source of phosphorus. Phosphorus, phosphates and phosphoric acid are still obtained from phosphate rock. Phosphate rock is a major feedstock in the fertilizer industry.
Bonding in phosphorus
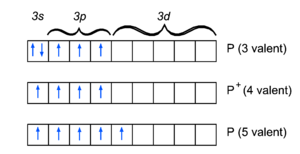
Since phosphorus is just below nitrogen in the periodic table it shares many of its bonding characteristics. For instance the molecule PH3 is the analogue of ammonia, NH3. Phosphorous, as nitrogen, is trivalent in this molecule,
This is the pre-quantum Lewis structure, which although somewhat of a simplification from a quantum chemical point of view[1], illustrates some of the distinguishing characteristics. The unpaired electrons in the 3p orbitals bind with those in the hydrogen 1s orbitals to form electron pairs of opposite spin. The electron pair in the phosphorus 3s orbital shows up as a lone pair in the Lewis structure.
Note
- ↑ In quantum chemical valence bond theory one usually works with mixtures of s and p atomic orbitals, so-called hybrids