Water
In common usage 'water' refers to the liquid substance that makes up the bulk of the oceans, lakes and rivers, the liquid that we and other animals, and plants, must take into our bodies to keep them living, and the liquid we use to wash our bodies and our clothes and put out fires. We learn early in life that we can transform liquid water into a solid water — ice — by 'freezing' it, and into a gas — steam, water vapor — by boiling it or allowing it to evaporate, and we come to realize that such transformations occur in naturally, accounting for glaciers, icy roads, and humid weather.
To scientists, 'water' refers to a particular molecule, and to collections of that molecule, each molecule consisting of the binding together in a particular way the atoms of two of the ninety-two naturally occurring chemical elements, oxygen and hydrogen — two hydrogen atoms binding to one oxygen atom per water molecule. A scientist specializing in a particular discipline (e.g., physics, chemistry, geology, biology) studies, describes and/or applies the characteristics of water particular her discipline.
This article approaches those particulars in a multi-disciplinary, cross-disciplinary and inter-disciplinary way.
The earth sciences perspective
Among the many subjects dealt with by the earth sciences, water presents itself as a major focus and challenge. In its broadest focus it deals with the distribution and masses of water on Earth. 'Oceanography' deals with water in the oceans; 'hydrology', with water on land and underground; 'meterology', with the characteristics of water in the Earth's atmosphere; 'glaciology', with water as glaciers and polar ice; 'climatology', with the role of water in Earth's climate. Earth scientists also study and teach about the history of water in Earth's history as a planet, and unavoidably, about the possibility of water elsewhere in the Universe.
Water is one of the Earth's basic naturally occurring substances. It covers about 70% of the world's surface…
The chemistry perspective
Chemistry deals with the properties of the water molecule itself and its interactions with other water molecules, and the way those properties and interactions account for its characteristics as 'water'.
The water molecule and its properties
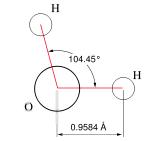
On a molecular level, water is a chemical compound comprised of two atoms of hydrogen and one atom of oxygen (H2O) (see figures at left and right).
Aggregates of water molecules and their properties
When heated to 100 degrees Celcius (its boiling point), water begins to convert to steam, and when cooled to 0 degrees Celcius (the freezing point) it converts to ice. Water is unique in that it is the only naturally occurring substance that is found in these three states.
When cooled down to 0 K (absolute zero) or as close as can be practically achieved water is the only known substance (as of this date) that has a rest vibrational energy. No other known substance exhibits this peculiar behavior as it was assumed that at the absolute zero temperature every molecule would be totally frozen, having an energy of 0 J/mol.
Additionally, water is usually referred to as "the universal solvent" because of its ability to dissolve more substances than any other existing liquid. It has a neutral acidity, which on a Ph scale has a value of 7.
Frozen water has over 20 crystal structures it can assume dependent upon the circumstances. These water structures are denoted by Roman numbers, I -- XX. The structures of the different ice crystals are reflecting the response of water and its intramolecular interactions to the environment while freezing. This behavior also is specific for water, again making it in this respect an astonishing chemical.
Another special behavior of water is its internal chargedistribution. Water is a polar liquid because of the electronegativity of O2 compared to [Hydrogen|H2]] making the Oxygen atom slightly negatively charged compared to the 2 Hydrogen atoms in the water molecule. Since the electron clouds around Oxygen are pyramidically shaped (compare visual with the diamond structure or the structure of methane) Osygen can form a pyramidical temporary bond with adjacent water molecules. These quickly forming and dissolving bonds are called hydrogen-bridges because they are energetically substantial yet are formed and broken at a very high rate. These hydrogen-bridges are important in the behavior of water (in all phases). It is the main reason for the high boiling point (373.14 K) and the low freezing point (273.14 K) as well as the exceptional behavior that the density of water is highest at 277.14 K (4˚C) where other liquid chemicals have the highest density at their freezing point.
The exceptional capability of water to dissolve very many chemicals is for a large part due to the structure water can form, breaking and forming hydrogen-bridges all the time and is the major contributor to the biological importance of water.
The biology perspective
Water by definition
The word "water" itself is practically synonymous with the word "liquid", as we refer to different liquids as water-like: "watered down", or "watery". We know that water moves and flows and is a force; to come across another liquid which visibly resembled water with an unknown chemical makeup, we might infer that it is water but would not know until more evidence was discovered.
Uses
The availability of water on the Earth affords humanity an incredible number of uses, aside from consumption as an integral part of survival. Water can be used to cool machinery and facilities such as nuclear power plants and industrial milling tools. Sometimes isotopes of hydrogen are used for that purpose: Deuterium, which is also known as "heavy water". For obvious reasons not the radioactive Tritium, the third isotope of Hydrogen. Water can also be heated to generate power--the focus of the steam engine which was born out of the industrial revolution, and hydroelectric dams which use water flow and gravity to turn turbines and rotors to generate electricity. Water can also be pressurized, creating a narrow stream that can cut through concrete and steel.
Treatment
In some areas of the United States, water is obtained from a dug well, which draws from a deep pocket under the surface called an aquafer. Areas that use water from shallow aquafers for irrigation may exhibit a distinct rust discoloration due to iron oxidation. However, digging deeper may produce consumable water. The availability of water below the earths surface and the depth at which it can be reached depends on the water table, which is directly impacted by the geological structure underneath.
Most of the water we consume is treated at a Water Treatment Plant. It is combined with levels of arsenic, flouride, and other additives in order to reduce potential malnutrients that might enter the water system and in the case of flouride, to help prevent tooth decay.
In recent decades a market for "bottled water" has developed. This water is often advertised to have been additionally filtered, or to have come from a spring in order to enhance it's "purity". The benefit of this bottled water has been disputed for a long time. Many governments are questioning the pollution created by the plastic bottles and are considering a ban on these pastic throw away bottles.. Other types of water sold may have additives such as flavors, caffeine, or natural herbal supplements.