User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok No edit summary |
imported>Milton Beychok |
||
| Line 23: | Line 23: | ||
==Calculation of acid dewpoints== | ==Calculation of acid dewpoints== | ||
The acid dewpoints of the five acids most commonly found in typical combustion product flue gases can be determined by using these equations: | |||
'''''Sulfuric acid (H<sub>2</sub>SO<sub>4</sub>) dew point:''''' <ref>{{cite journal|author=F.H. Verhoff and J.T. Banchero|title=Predicting Dew Points of Gases |journal=Chemical Engineering progress|volume=78|issue=8|pages=71 - 72|date=1974|id=|url=}}</ref><ref>{{cite journal|author=R.R. Pierce|title=Estimating Acid Dewpoints in Stack Gases|journal=Chemical Engineering|volume=84|issue=8|pages=125 - 128|date=1977|id=|url=}}</ref> | '''''Sulfuric acid (H<sub>2</sub>SO<sub>4</sub>) dew point:''''' <ref>{{cite journal|author=F.H. Verhoff and J.T. Banchero|title=Predicting Dew Points of Gases |journal=Chemical Engineering progress|volume=78|issue=8|pages=71 - 72|date=1974|id=|url=}}</ref><ref>{{cite journal|author=R.R. Pierce|title=Estimating Acid Dewpoints in Stack Gases|journal=Chemical Engineering|volume=84|issue=8|pages=125 - 128|date=1977|id=|url=}}</ref> | ||
| Line 43: | Line 45: | ||
:(5) <math>1000/T = 3.6614\, -\, 0.1446\, \log_e\,(P_\mathrm{H_2O})\, -\, 0.0827\, \log_e\,(P_\mathrm{HNO_3})\, +\, 0.00756\, \log_e\,(P_\mathrm{H_2O})\, \log_e\,(P_\mathrm{HNO_3})</math> | :(5) <math>1000/T = 3.6614\, -\, 0.1446\, \log_e\,(P_\mathrm{H_2O})\, -\, 0.0827\, \log_e\,(P_\mathrm{HNO_3})\, +\, 0.00756\, \log_e\,(P_\mathrm{H_2O})\, \log_e\,(P_\mathrm{HNO_3})</math> | ||
where: | |||
:{|border="0" cellpadding="2" | |||
|- | |||
|align=right|<math>T</math> | |||
|align=left|= The acid dewpoint temperature for the indicated acid, ( K ) | |||
|- | |||
|align=right|<math>P</math> | |||
|align=left|= Partial pressure, ( atm for equation 1 and mmHg for equations 2, 3, 4 and 5 ) | |||
|} | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
Revision as of 00:22, 22 May 2010
The acid dew point (also acid dewpoint) of a flue gas (i.e., a combustion product gas) is the temperature, at a given pressure, at which any gaseous acid in the flue gas will start to condense into liquid acid.[1][2][3]
The acid dew point of a flue gas, at a given pressure, is often referred to as the point at which the flue gas is "saturated" with gaseous acid, meaning that the flue gas cannot hold any more gaseous acid.
In many industrial combustion processes, the flue gas is cooled by the recovery of heat from the hot flue gases before they are emitted to the atmosphere from the final flue gas stack (commonly referred to as a chimney). It is very important not to cool the flue gas below its acid dewpoint because the resulting liquid acid condensed from the flue gas can cause serious corrosion problems for the equipment used in transporting, cooling and emitting the the flue gas.
Chemistry and mechanism
Sulfuric acid dewpoint
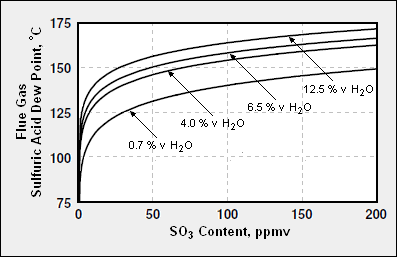
Calculated sulfuric acid dew points of typical combustion flue gases, as a function of SO3 content, and water vapor content [4]
As a broad generality, flue gases from the combustion of coal, fuel oil, natural gas, or biomass are primarily composed of carbon dioxide (CO2) and water vapor (H2O) as well as nitrogen (N2) and excess oxygen (O2) remaining from the intake combustion air. Typically, more than two-thirds of the flue gas is nitrogen. The combustion flue gases may also contain small amounts of particulate matter, carbon monoxide, nitrogen oxides, and sulfur oxides in the form of gaseous sulfur dioxide (SO2) and gaseous sulfur trioxide (SO3). The SO3 is present because a portion of the SO2 formed in the combustion of the sulfur (S) compounds in the combustion fuel is further oxidized to SO3. The gas phase SO3 then combines the vapor phase H2O to form gas phase sulfuric acid H2SO4:
- H2O + SO3 → H2SO4
- water + sulfur trioxide → sulfuric acid
Because of the presence of gaseous sulfuric acid, the sulfuric acid dew point of most flue gases is much higher than the water dew point of the flue gases. For example, a flue gas with 5 volume % water vapor and containing no acid gases has a water dew point of about 32 °C (90 °F). The same flue gas with the addition of only 0.01 volume percent of SO3 will have a sulfuric acid dew point of about 118 °C (244 °F).[5]
The acid dew point of a combustion flue gas depends upon the composition of the specific fuel being burned and the resultant composition of the flue gas. Given a flue gas composition, its acid dew point can be predicted fairly closely. As an approximation, the sulfuric acid dew points of flue gases from thermal power plants range from about 120 °C to about 150 °C (250 to 300 °F).
Other acid dewpoints
Calculation of acid dewpoints
The acid dewpoints of the five acids most commonly found in typical combustion product flue gases can be determined by using these equations:
Sulfuric acid (H2SO4) dew point: [6][7]
- (1)
- or this equivalent form:
- (2)
Sulfurous acid (H2SO3) dew point: [8]
- (3)
Hydrochloric acid (HCl) dewpoint: [8]
- (4)
Nitric acid (HNO3) dewpoint: [8]
- (5)
where:
= The acid dewpoint temperature for the indicated acid, ( K ) = Partial pressure, ( atm for equation 1 and mmHg for equations 2, 3, 4 and 5 )
References
- ↑ David A. Lewandowski (2000). Design of Thermal Oxidation Systems for Volatile Organic Compounds, 1st Edition. CRC Press. ISBN 1-56670-410-3.
- ↑ Walter R. Niessen (2002). Combustion and Incineration Processes, 3rd Edition. CRC Press. ISBN 0-8247-0629-3.
- ↑ W.M.M. Huijbregts and R. Leferink (2004). "Latest Advances in the Understanding of Acid Dewpoint Corrosion: Corrosion and Stress Corrosion Cracking in Combustion Gas Condensates". Anti-Corrosion Methods and Materials 51 (3): 173 - 188.
- ↑ Condensing Economizer Article
- ↑ Burning Sulfur Compounds A publication of the Banks Engineering Company of Oklahoma.
- ↑ F.H. Verhoff and J.T. Banchero (1974). "Predicting Dew Points of Gases". Chemical Engineering progress 78 (8): 71 - 72.
- ↑ R.R. Pierce (1977). "Estimating Acid Dewpoints in Stack Gases". Chemical Engineering 84 (8): 125 - 128.
- ↑ 8.0 8.1 8.2 Yen Hsiung Kiang (1981). "Predicting Dewpoints of Gases". Chemical Engineering 88 (3): 127.






