Inositol: Difference between revisions
imported>David E. Volk |
mNo edit summary |
||
| Line 8: | Line 8: | ||
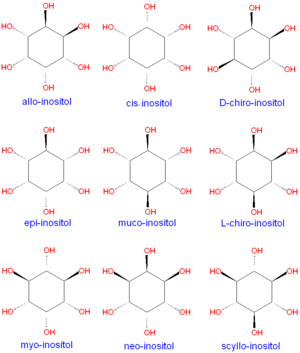
{{Image|Inositol stereo isomers.png|left|300px|Natural inositol forms.}} | {{Image|Inositol stereo isomers.png|left|300px|Natural inositol forms.}} | ||
==Stereochemistry of inositol == | ==Stereochemistry of inositol == | ||
Although myo-inositol is the most common form of inositol formed in nature, many other stereo isomers are known, as shown below. Only two of the inositol forms, D-''chiro''-inositol and L-''chiro''-inositol, are enantiomers that are optically active. | Although myo-inositol is the most common form of inositol formed in nature, many other stereo isomers are known, as shown below. Only two of the inositol forms, D-''chiro''-inositol and L-''chiro''-inositol, are enantiomers that are optically active.[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:01, 1 September 2024
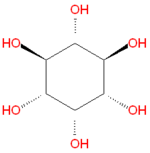
Inositol refers to a collection hexose carbohydrates based on a central cyclohexane structure with six hydroxyl (OH) groups. Although myo-inositol, previously called meso-inositol, is the most common version found in nature, a number of other stereoisomeric forms are produced naturally. Inositol acts as a fatty acid transport by coupling its hydroxyl oxygen atoms with the carboxyl groups of fatty acids. The stereochemistry of myo-inositol is denoted by the IUPAC name cis-1,2,3,5-trans-4,6-cyclohexanehexol.
Stereochemistry of inositol
Although myo-inositol is the most common form of inositol formed in nature, many other stereo isomers are known, as shown below. Only two of the inositol forms, D-chiro-inositol and L-chiro-inositol, are enantiomers that are optically active.