User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok (→Scales) |
imported>Milton Beychok (→Scales) |
||
| Line 22: | Line 22: | ||
:Note: 60 [[Fahrenheit (unit)|°F]] is equivalent to 15.56 °C. | :Note: 60 [[Fahrenheit (unit)|°F]] is equivalent to 15.56 °C. | ||
;[[Baumé gravity]]: These two scales, one for liquids lighter than water and one for liquids heavier than water, were developed by the [[France|French]] chemist [[Antoine Baumé]] in 1768. It is widely used in industrial chemistry, [[pharmacology]], [[sugar refining]] and other industries. The two scales are are expressed as: | ;[[Baumé gravity]]: These two scales, one for liquids lighter than water and one for liquids heavier than water, were developed by the [[France|French]] chemist [[Antoine Baumé]] in 1768. It is widely used in industrial chemistry, [[pharmacology]], [[sugar refining]] and other industries. The two scales are are expressed as:<ref name=Perry's>{{cite book|author=Perry, R.H. and Green, D.W.|title=Perry's Chemical Engineers' Handbook |edition=6th Edition| publisher=McGraw Hill, Inc.|year=1984|pages=page 1-19|id=ISBN ISBN 0-07-049479-7}}</ref><ref>[http://antoine.frostburg.edu/chem/senese/101/measurement/faq/baume-scale.shtml What is a "degree Baume'?] Professor Frederick A. Senese, Chemistry Department, [[Frostburg State University]], [[Maryland]]</ref> | ||
:<math>^\circ \mathrm{B\acute{e}} = \frac{140}{\mathrm{SG}_{20\,^\circ \mathrm{C}}^{20\,^\circ \mathrm{C}}} - \mathrm{130}</math> for liquids lighter than water and <math>\mathrm{SG}_{20\,^\circ \mathrm{C}}^{20\,^\circ \mathrm{C}} = \frac {\mathrm{density\; of\; the\; tested\; liquid\; at\; 20\,^\circ \mathrm{C}}} {\mathrm{density\; of\; water\; at\; 20\,^\circ \mathrm{C}}}</math> | :<math>^\circ \mathrm{B\acute{e}} = \frac{140}{\mathrm{SG}_{20\,^\circ \mathrm{C}}^{20\,^\circ \mathrm{C}}} - \mathrm{130}</math> for liquids lighter than water and <math>\mathrm{SG}_{20\,^\circ \mathrm{C}}^{20\,^\circ \mathrm{C}} = \frac {\mathrm{density\; of\; the\; tested\; liquid\; at\; 20\,^\circ \mathrm{C}}} {\mathrm{density\; of\; water\; at\; 20\,^\circ \mathrm{C}}}</math> | ||
| Line 44: | Line 44: | ||
;[[Twaddell gravity]]: | ;[[Twaddell gravity]]: | ||
In the 19th centuery, this scale was developed in [[Glasgow]], [[Scotland]] by [[William Twaddell]], an instrument maker.<ref>[http://nms.scran.ac.uk/database/record.php?usi=000-190-004-180-C Hydrometers] From the website of the [[National Museums of Scotland]].</ref> It is used in a number of industries and it is expressed as:<ref | In the 19th centuery, this scale was developed in [[Glasgow]], [[Scotland]] by [[William Twaddell]], an instrument maker.<ref>[http://nms.scran.ac.uk/database/record.php?usi=000-190-004-180-C Hydrometers] From the website of the [[National Museums of Scotland]].</ref> It is used in a number of industries and it is expressed as:<ref name=Perry's/> | ||
:<math>^\circ \mathrm{Tw} = 200\, (\mathrm{SG}_{60\,^\circ \mathrm{F}}^{60\,^\circ \mathrm{F}} -1)</math> | :<math>^\circ \mathrm{Tw} = 200\, (\mathrm{SG}_{60\,^\circ \mathrm{F}}^{60\,^\circ \mathrm{F}} -1)</math> | ||
Revision as of 16:55, 3 February 2010
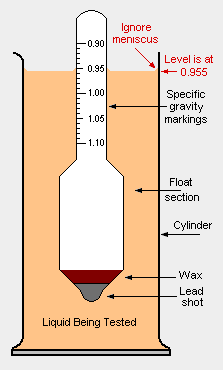
A hydrometer is an instrument used to measure the specific gravity (SG) (or relative density) of liquids; that is, the ratio of the density of the liquid to the density of water with both at the same temperature. It is usually made of glass and consists of a small diameter cylindrical stem and a larger diameter section weighted with mercury or lead shot (sealed with wax) to make it float upright. The larger diameter section is necessary to provide the displacement volume needed for the proper buoyancy of the hydrometer
The stem contains a rolled paper marked with the scale being used. There are a great many different hydrometer scales commonly used to measure liquid densities in: petroleum crude oil marketing and refining; making wine, brewing beer and making whiskey; refining sugar; producing sulfuric acid and other industrial chemicals.
Scales
The many hydrometer scales that are commonly used include:
- Specific gravity (SG)
- This scale is widely used in chemistry, engineering and physics as well as by geologists, mineralogists and gemologists. It is often referred to as relative density and it is the one shown in the adjacent drawing. Specific gravity is expressed as:
- Note 1: Unless the two reference temperatures are explicitly stated, they are generally taken to be 4 °C.
- Note 2: The specific gravity of solids can be determined by using a pycnometer rather than a hydrometer.
- API gravity
- This scale was developed by the American Petroleum Institute (API) in 1921 for use in the petroleum industry and it is now universally used by the petroleum industry worldwide. It is expressed as:[1]
- and
- Note: 60 °F is equivalent to 15.56 °C.
- Baumé gravity
- These two scales, one for liquids lighter than water and one for liquids heavier than water, were developed by the French chemist Antoine Baumé in 1768. It is widely used in industrial chemistry, pharmacology, sugar refining and other industries. The two scales are are expressed as:[2][3]
- for liquids lighter than water and
- for liquids heavier than water and
- Brix gravity
- This scale was developed in the 1854 by Adolf Ferdinand Wenceslaus Brix, a German or Austrian engineer and mathematician. It is widely used in beer brewing, wineries, sugar refining and fruit juice industry. It is expressed as:
- and
- Oechsle gravity
- This scale was developed in the 1830's by Christian Ferdinand Oechsle, a German pharmacist and goldsmith. It is used in Germany, Austria and Switzerland in wineries and beer brewing. It is expressed as:
- Plato gravity
- This scale was developed in 1918 by Dr. Fritz Plato , a German scientist. It is primarily used in the beer brewing industry and it is expressed as:
- and
The origin of the Plato scale lies in the Balling Scale developed in 1835 by Carl Joseph Napoleon Balling which was recalibrated by Brix in 1854 and renamed the Brix scale. In 1918, Dr. Plato then developed his scale by improving and correcting Balling's original work. Basically, the Balling, Brix and Plato scales are identical up to the fifth and sixth decimal place.[4]
In the 19th centuery, this scale was developed in Glasgow, Scotland by William Twaddell, an instrument maker.[5] It is used in a number of industries and it is expressed as:[2]
- Others
- The leather tanning industry uses a Barkometer that expresses specific gravity in Barkometer degrees.
- The dairy industry uses a Lactometer calibrated in Quevenne degrees in testing milk.
- The alcohol industry uses the Sikes, Richter, or Tralles scales on their Alcoholometers. Each of them reads the volumetric percentage of ethyl alcohol in water.
History
Knowledge of relative density or specific gravity has been with us since the days of Archimedes in 250 BC, with the observation that light objects can float while heavier ones will sink in water.[6] Hypatia (born ca. 350 and died 415 AD), a Greek scholar from Alexandria in Egypt and considered to be the first notable female mathematician, is reputed to have invented the hydrometer.[7]
Several key figures in the history of science have mentioned the hydrometer in their work, including Galileo in 1612. In the 18th and 19th centuries, industrial development in Europe spurred the need for the hydrometer. It gained fame due to public controversy over alcohol taxation since the hydrometer was used in the distillation industry to measure alcohol content and determine excise taxes in England.
A great deal more detailed history is provided in the book, edited by Holmes and Lever, about the history of instruments in chemistry from the days of the alchemists through the creation of the modern chemistry laboratory.[8]
References
- ↑ API Gravity References the publication: Ernest L. Ruh, James J. Moran and Robert D. Thompson (1959). Measurement problems in the instrument and laboratory apparatus fields. American Association for the Advancement of Science (AAAS), Page 29. AAAS Publication No. 57.
- ↑ 2.0 2.1 Perry, R.H. and Green, D.W. (1984). Perry's Chemical Engineers' Handbook, 6th Edition. McGraw Hill, Inc., page 1-19. ISBN ISBN 0-07-049479-7.
- ↑ What is a "degree Baume'? Professor Frederick A. Senese, Chemistry Department, Frostburg State University, Maryland
- ↑ Peter Hull (2010). Glucose Syrups: Technology and Applications, 1st Edition. Wiley-Blackwell. ISBN 1-4051-7556-7. Chapter 1 is available online at Chapter 1, History of Glucose Syrups]
- ↑ Hydrometers From the website of the National Museums of Scotland.
- ↑ T. L. Heath (1897). The Works of Archimedes. Cambridge University Press, page 253. Full text available at www.archive.org
- ↑ Mothers of Invention Ethlie Ann Vare and Greg Ptacek, 1988, pp. 24-26.
- ↑ Freerick L. Holmes and Trevor H. Levere (Editors) (2002). Instruments and Experimentation in the History of Chemistry, 1st Edition, 2nd Printing. The MIT Press. ISBN 0-262-08282-9.