Fibromyalgia: Difference between revisions
imported>Robert Badgett |
imported>Robert Badgett |
||
| Line 116: | Line 116: | ||
[[Pregabalin]] (Lyrica) is approved for use in the United States by the [[Food and Drug Administration]] (FDA) for fibromyalgia. As compared to placebo, [[pregabalin]] benefits about 1 of every 6 people who use it if they are similar to the patients in a [[randomized controlled trial]]. In this trial, 33% of the patients who took the drug had a reduction in their pain versus 13% of the patients who took placebo.<ref name="pmid15818684">{{cite journal |author=Crofford LJ, Rowbotham MC, Mease PJ, ''et al'' |title=Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial |journal=Arthritis Rheum. |volume=52 |issue=4 |pages=1264–73 |year=2005 |pmid=15818684 |doi=10.1002/art.20983}}</ref> In a second [[randomized controlled trial]], 43% of the patients who took the drug had a reduction in their pain versus 35% of the patients who took placebo. In this second trial, one out of every twelve patients taking the drug benefited from the drug. <ref name="pmid18278830">{{cite journal |author=Mease PJ, Russell IJ, Arnold LM, ''et al'' |title=A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia |journal=The Journal of rheumatology |volume=35 |issue=3 |pages=502–14 |year=2008 |month=March |pmid=18278830 |doi= |url=http://www.jrheum.com/subscribers/08/03/502.html |issn=}}</ref> Both trials were sponsored by Pfizer, the makers of Lyrica, which increases the likelihood of [[publication bias]] towards positive results. | [[Pregabalin]] (Lyrica) is approved for use in the United States by the [[Food and Drug Administration]] (FDA) for fibromyalgia. As compared to placebo, [[pregabalin]] benefits about 1 of every 6 people who use it if they are similar to the patients in a [[randomized controlled trial]]. In this trial, 33% of the patients who took the drug had a reduction in their pain versus 13% of the patients who took placebo.<ref name="pmid15818684">{{cite journal |author=Crofford LJ, Rowbotham MC, Mease PJ, ''et al'' |title=Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial |journal=Arthritis Rheum. |volume=52 |issue=4 |pages=1264–73 |year=2005 |pmid=15818684 |doi=10.1002/art.20983}}</ref> In a second [[randomized controlled trial]], 43% of the patients who took the drug had a reduction in their pain versus 35% of the patients who took placebo. In this second trial, one out of every twelve patients taking the drug benefited from the drug. <ref name="pmid18278830">{{cite journal |author=Mease PJ, Russell IJ, Arnold LM, ''et al'' |title=A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia |journal=The Journal of rheumatology |volume=35 |issue=3 |pages=502–14 |year=2008 |month=March |pmid=18278830 |doi= |url=http://www.jrheum.com/subscribers/08/03/502.html |issn=}}</ref> Both trials were sponsored by Pfizer, the makers of Lyrica, which increases the likelihood of [[publication bias]] towards positive results. | ||
[[Duloxetine]] (Cymbalta) | [[Duloxetine]] (Cymbalta), a SNRI [[second-generation antidepressant]], is approved for use in the United States by the [[Food and Drug Administration]] (FDA) for fibromyalgia. As compared to placebo, [[duloxetine]] 60 mg per day benefits about 1 of every 7 people who use it if they are similar to the patients in an [[randomized controlled trial]]. In this trial, 51% of the patients who took the drug had a ''30%'' reduction in their pain versus 36% of the patients who took placebo (data not reported for 50% reduction).<ref name="pmid18395345">{{cite journal |author=Russell IJ, Mease PJ, Smith TR, ''et al'' |title=Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: Results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial |journal=Pain |volume=136 |issue=3 |pages=432–44 |year=2008 |month=June |pmid=18395345 |doi=10.1016/j.pain.2008.02.024 |url=http://linkinghub.elsevier.com/retrieve/pii/S0304-3959(08)00114-0 |issn=}}</ref> This trial was sponsored by the makers of Cymbalta which increases the likelihood of [[publication bias]] towards positive results. | ||
[[Milnacipran]], a SNRI [[second-generation antidepressant]] is approved for use in the United States by the [[Food and Drug Administration]] (FDA) for fibromyalgia.<ref name="urlThe Medical Letter on Drugs and Therapeutics">{{cite web |url=http://www.medicalletter.org/restricted/articles/w1314b.html |title=Milnacipran (Savella) for Fibromyalgia |author=Anonymous |authorlink= |coauthors= |date=2009 |format= |work= |publisher=The Medical Letter on Drugs and Therapeutics |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=}}</ref> | |||
====New therapies==== | ====New therapies==== | ||
Revision as of 01:04, 15 June 2009
Fibromyalgia is one of a variety of disorders categorized as chronic widespread pain by the American College of Rheumatology. "Muscle pain is typically aggravated by inactivity or exposure to cold. This condition is often associated with general symptoms, such as sleep disturbances, fatigue, stiffness, headaches, and occasionally depression. There is significant overlap between fibromyalgia and the chronic fatigue syndrome (fatigue syndrome, chronic). Fibromyalgia may arise as a primary or secondary disease process. It is most frequent in females aged 20 to 50 years." (From Adams et al., Principles of Neurology, 6th ed, p1494-95)".[1]
Multiple comorbid symptoms and syndromes may accompany fibromyalgia, including tension/migraine headache, affective disorders, temporomandibular joint disorder, idiopathic low back pain, irritable bowel syndrome, nondermatomal paresthesias, fatigue, memory and cognitive difficulties, and others. Comorbid tension headache is present in over 70% of women and 50% of men with fibromyalgia.
Once a diagnosis concerned, the next steps involve identification, in this highly individual disease, of important symptom domains, their severity, and level of patient function. The only diagnostic instrument that has been validated for measure of function and quality of life in fibromyalgia is the Fibromyalgia Impact Questionnaire (FIQ). Patients should be evaluated for comorbid medical and psychiatric disorders, psychosocial stressors, level of fitness, and barriers to treatment. Education should be provided and treatment options should be reviewed.
Etiology/causation
Fibromyalgia appears to have a multifactorial pathophysiology, which includes a strong familial predisposition, central pain amplification, psychiatric comorbid conditions, and other factors, such as immune dysregulation and the role of neurohormones such as dopamine, and growth hormone. Magnetic resonance imaging studies have provided objective evidence to show that patients with fibromyalgia have a lower threshold for pain sensitivity.[2]
Genetics
Using self-report of Chronic Widespread Pain (CWP) as a surrogate marker for fibromyalgia, the Swedish Twin Registry suggests a modest genetic contribution:[3][4]
- Monozygotic twins with CWP have a 15% chance that their twin sibling has CWP
- Dizygotic twins with CWP have a 7% chance that their twin sibling has CWP
Abnormal sleep
Fibromyalgia is associated with alpha sleep.[5] However, it is unclear if this abnormal sleep pattern causes or follows fibromyalgia.
Vitamin D deficiency
Although initial studies suggest that low vitamin D levels may be associated with nonspecific musculoskeletal pain[6]; more recent studies make this doubtful.[7][7]
Symptoms
Symptoms from associated illnesses
Fluid retention might be associated with fibromyalgia.[8][9] This is difficult to interpret as normal patients may have a 9 pound diurnal variation in weight.[10]
Diagnosis
American College of Rheumatology diagnostic criteria
Specific ACR diagnostic criteria are:[11]
- History of widespread pain, considered widespread when all of the following are present: pain in the left and right sides of the body, pain above and below below the waist, and axial skeletal pain (cervical spine or anterior chest or thoracic spine or low back). In this definition, shoulder and buttock pain is considered as pain for each involved side. "Low back" pain is considered lower segment pain.
- An examiner must be able to trigger what the patient describes as "pain", not "tenderness", with finger pressure of approximately 4 kg, at 11 of the following 18 sites:
- Occiput: Bilateral, at the suboccipital muscle insertions.
- Low cervical [spine]: bilateral, at the anterior aspects of the intertransverse spaces at C5-C7.
- Trapezius muscle: bilateral, at the midpoint of the upper border.
- Supraspinatus muscle: bilateral, at origins, above the scapula spine near the medial border.
- Second rib: bilateral, at the second costochondral junctions, just lateral to the junctions on upper surfaces.
- Lateral epicondyle: bilateral, 2 cm distal to the epicondyles.
- Gluteal: bilateral, in upper outer quadrants of buttocks in anterior fold of muscle.
- Greater trochanter: bilateral, posterior to the trochanteric prominence.
- Knee: bilateral, at the medial fat pad proximal to the joint line.
The ACR diagnostic criteria have accuracy of:[12]
- Sensitivity 88.4%
- Specificity 81.1%
Differential diagnosis
A cohort study found that widespread pain significantly increased risk of subsequent diagnosis of cancer, especially breast cancer and prostate cancer.[13] The biological reason for this association is not clear.
Treatment
Clinical practice guidelines by the European League Against Rheumatism (EULAR) recommendations (parentheses contain levels of evidence and strength of recommendation) are included below.[14]
General
- "Full understanding of fibromyalgia requires comprehensive assessment of pain, function and psychosocial context. Fibromyalgia should be recognised as a complex and heterogeneous condition where there is abnormal pain processing and other secondary features" (IV D)
- "Optimal treatment requires a multidisciplinary approach with a combination of non-pharmacological and pharmacological treatment modalities tailored according to pain intensity, function, associated features such as depression, fatigue and sleep disturbance in discussion with the patient" (IV D)
Despite different modes of action, a variety of neuromodulatory agents may improve the symptoms of fibromyalgia patients. These include antidepressants, analgesics, anticonvulsants, muscle relaxants, and sedative hypnotic drugs. Only 2 drugs are FDA-approved for the specific treatment of fibromyalgia: pregabalin (Lyrica®) and duloxetine hydrochloride (Cymbalta®).[12]
Non-pharmacological management
According to the clinical practice guidelines:[14]
- "Heated pool treatment with or without exercise is effective in fibromyalgia" (IIa B)
- "Individually tailored exercise programmes, including aerobic exercise and strength training can be beneficial to some patients" with fibromyalgia" (IIb C). Subsequent to the 2008 EULAR clinical practice guidelines, a meta-analysis of randomized controlled trials found "aerobic-only training has beneficial effects on physical function and some FM symptoms. Strength-only training may improve FM symptoms, but requires further study."[15]
- "Cognitive behavioural therapy may be of benefit to some patients with fibromyalgia" IV D)
- "Other therapies such as relaxation, rehabilitation, physiotherapy and psychological support may be used depending on the needs of the individual patient (IIb C)
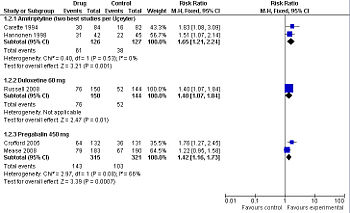
Pharmacological management
| Study | Patients | Intervention | Results |
|---|---|---|---|
| Amitriptyline | |||
| Üçeyler, 2008[16] Meta-analysis |
multiple trials | Various doses of 10-100 mg per day | Did not pool comparative outcomes |
| Arnold, 2000[17] Meta-analysis |
multiple trials | Various doses of 5-50 mg per day | Did not pool comparative outcomes |
| O'Malley, 2000[18] Meta-analysis |
multiple trials | Various doses of 5-50 mg per day | Did not isolate the effect of amitriptyline |
| Carette, 1994[19] Randomized controlled trial |
166 patients | 10 to 50 mg per day | response at 6 months drug = 36% and placebo = 19% |
| Hannonen, 1998[20] Randomized controlled trial |
97 patients | 25 mg or 37.5 mg per day | At least minimal improvement: response at 3 months drug = 74% and placebo = 49% |
| Duloxetine | |||
| Arnold, 2004[21] Randomized controlled trial |
207 patients | 60 mg twice a day | No dichotomous results reported |
| Russell, 2008[22] Randomized controlled trial |
194 patients | duloxetine 60 mg | 30% reduction in their pain: response at 3 months, drug = 51% and placebo = 36% |
| Pregabalin | |||
| Mease, 2008[23] Randomized controlled trial |
373 patients | pregabalin 450 mg | At least "much" improvement in their pain, drug = 43% and placebo = 35% At least "minimal" improvement in their pain, drug = 71% and placebo = 56% |
| Crofford, 2005[24] Randomized controlled trial |
263 patients | pregabalin 450 mg | 30% reduction in their pain: response at 3 months, drug = 48% and placebo = 27% |
According to the clinical practice guidelines:[14]
- "Tramadol is recommended for the management of pain in fibromyalgia" (Ib A)
- "Simple analgesics such as paracetamol and other weak opioids can also be considered in the treatment of fibromyalgia. Corticosteroids and strong opioids are not recommended (IV D)
- "Antidepressants: amitriptyline, fluoxetine, duloxetine, milnacipran, moclobemide and pirlindole, reduce pain and often improve function, therefore they are recommended for the treatment of fibromyalgia" (Ib A)
- Tropisetron, pramipexole and pregabalin reduce pain and are recommended for the treatment of fibromyalgia" (Ib A)
Pregabalin (Lyrica) is approved for use in the United States by the Food and Drug Administration (FDA) for fibromyalgia. As compared to placebo, pregabalin benefits about 1 of every 6 people who use it if they are similar to the patients in a randomized controlled trial. In this trial, 33% of the patients who took the drug had a reduction in their pain versus 13% of the patients who took placebo.[24] In a second randomized controlled trial, 43% of the patients who took the drug had a reduction in their pain versus 35% of the patients who took placebo. In this second trial, one out of every twelve patients taking the drug benefited from the drug. [23] Both trials were sponsored by Pfizer, the makers of Lyrica, which increases the likelihood of publication bias towards positive results.
Duloxetine (Cymbalta), a SNRI second-generation antidepressant, is approved for use in the United States by the Food and Drug Administration (FDA) for fibromyalgia. As compared to placebo, duloxetine 60 mg per day benefits about 1 of every 7 people who use it if they are similar to the patients in an randomized controlled trial. In this trial, 51% of the patients who took the drug had a 30% reduction in their pain versus 36% of the patients who took placebo (data not reported for 50% reduction).[22] This trial was sponsored by the makers of Cymbalta which increases the likelihood of publication bias towards positive results.
Milnacipran, a SNRI second-generation antidepressant is approved for use in the United States by the Food and Drug Administration (FDA) for fibromyalgia.[25]
New therapies
Sodium oxybate may help according to a randomized controlled trial that was funded by industry.[26]
References
- ↑ National Library of Medicine. Fibromyalgia. Retrieved on 2007-11-13.
- ↑ Abeles AM, Pillinger MH, Solitar BM, Abeles M (2007). "Narrative review: the pathophysiology of fibromyalgia". Ann. Intern. Med. 146 (10): 726–34. PMID 17502633. [e]
- ↑ Kato K, Sullivan P, Evengård B, Pedersen N (2006). "Importance of genetic influences on chronic widespread pain". Arthritis Rheum. 54 (5): 1682-6. DOI:10.1002/art.21798. PMID 16646040. Research Blogging.
- ↑ Kato K, Sullivan P, Evengård B, Pedersen N (2006). "Chronic widespread pain and its comorbidities: a population-based study". Arch. Intern. Med. 166 (15): 1649-54. PMID 16908799.
- ↑ Roizenblatt S, Moldofsky H, Benedito-Silva AA, Tufik S (2001). "Alpha sleep characteristics in fibromyalgia". Arthritis Rheum. 44 (1): 222–30. DOI:<222::AID-ANR29>3.0.CO;2-K 10.1002/1529-0131(200101)44:1<222::AID-ANR29>3.0.CO;2-K. PMID 11212164. <222::AID-ANR29>3.0.CO;2-K Research Blogging.
- ↑ Plotnikoff GA, Quigley JM (2003). "Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain". Mayo Clin. Proc. 78 (12): 1463–70. PMID 14661675. [e]
- ↑ 7.0 7.1 Warner AE, Arnspiger SA (February 2008). "Diffuse musculoskeletal pain is not associated with low vitamin D levels or improved by treatment with vitamin D". J Clin Rheumatol 14 (1): 12–6. DOI:10.1097/RHU.0b013e31816356a9. PMID 18431091. Research Blogging.
- ↑ Deodhar AA, Fisher RA, Blacker CV, Woolf AD (June 1994). "Fluid retention syndrome and fibromyalgia". Br. J. Rheumatol. 33 (6): 576–82. PMID 8205408. [e]
- ↑ Dunnigan MG, Henderson JB, Hole D, Pelosi AJ (November 2004). "Unexplained swelling symptoms in women (idiopathic oedema) comprise one component of a common polysymptomatic syndrome". QJM 97 (11): 755–64. DOI:10.1093/qjmed/hch126. PMID 15496531. Research Blogging.
- ↑ Denning DW, Dunnigan MG, Tillman J, Davis JA, Forrest CA (May 1990). "The relationship between 'normal' fluid retention in women and idiopathic oedema". Postgrad Med J 66 (775): 363–6. PMID 2371185. [e]
- ↑ Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. (1990), "The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicenter criteria committee.", Arthritis Rheum 33: 160-172
- ↑ 12.0 12.1 {{ title = Highlights of the American Academy of Pain Management 19th Annual Clinical Meeting: An Update on Chronic Pain Treatments | first = Andrew N. | last = Wilner | contribution = Management of Fibromyalgia | url = http://www.medscape.com/viewarticle/581929 | year = 2008}}
- ↑ McBeth J, Silman AJ, Macfarlane GJ (2003). "Association of widespread body pain with an increased risk of cancer and reduced cancer survival: a prospective, population-based study". Arthritis Rheum. 48 (6): 1686–92. DOI:10.1002/art.10973. PMID 12794837. Research Blogging.
- ↑ 14.0 14.1 14.2 Carville SF, Arendt-Nielsen S, Bliddal H, et al (2008). "EULAR evidence-based recommendations for the management of fibromyalgia syndrome". Ann. Rheum. Dis. 67 (4): 536-41. DOI:10.1136/ard.2007.071522. PMID 17644548. Research Blogging.
- ↑ Busch AJ, Schachter CL, Overend TJ, Peloso PM, Barber KA (June 2008). "Exercise for fibromyalgia: a systematic review". J. Rheumatol. 35 (6): 1130–44. PMID 18464301. [e]
- ↑ Uçeyler N, Häuser W, Sommer C (September 2008). "A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome". Arthritis and rheumatism 59 (9): 1279–98. DOI:10.1002/art.24000. PMID 18759260. Research Blogging.
- ↑ Arnold LM, Keck PE, Welge JA (2000). "Antidepressant treatment of fibromyalgia. A meta-analysis and review". Psychosomatics 41 (2): 104–13. PMID 10749947. [e]
- ↑ O'Malley PG, Balden E, Tomkins G, Santoro J, Kroenke K, Jackson JL (September 2000). "Treatment of fibromyalgia with antidepressants: a meta-analysis". Journal of general internal medicine : official journal of the Society for Research and Education in Primary Care Internal Medicine 15 (9): 659–66. PMID 11029681. PMC 1495596. [e]
- ↑ Carette S, Bell MJ, Reynolds WJ, et al (January 1994). "Comparison of amitriptyline, cyclobenzaprine, and placebo in the treatment of fibromyalgia. A randomized, double-blind clinical trial". Arthritis and rheumatism 37 (1): 32–40. PMID 8129762. [e]
- ↑ Hannonen P, Malminiemi K, Yli-Kerttula U, Isomeri R, Roponen P (December 1998). "A randomized, double-blind, placebo-controlled study of moclobemide and amitriptyline in the treatment of fibromyalgia in females without psychiatric disorder". British journal of rheumatology 37 (12): 1279–86. PMID 9973149. [e]
- ↑ Arnold LM, Lu Y, Crofford LJ, et al (September 2004). "A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder". Arthritis and rheumatism 50 (9): 2974–84. DOI:10.1002/art.20485. PMID 15457467. Research Blogging.
- ↑ 22.0 22.1 Russell IJ, Mease PJ, Smith TR, et al (June 2008). "Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: Results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial". Pain 136 (3): 432–44. DOI:10.1016/j.pain.2008.02.024. PMID 18395345. Research Blogging.
Clinical trial registration number NCT00190866 Cite error: Invalid
<ref>tag; name "pmid18395345" defined multiple times with different content - ↑ 23.0 23.1 Mease PJ, Russell IJ, Arnold LM, et al (March 2008). "A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia". The Journal of Rheumatology 35 (3): 502–14. PMID 18278830. [e]
Trial registration number not provided by authors, but is likely to be NCT00333866 Cite error: Invalid
<ref>tag; name "pmid18278830" defined multiple times with different content - ↑ 24.0 24.1 Crofford LJ, Rowbotham MC, Mease PJ, et al (2005). "Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial". Arthritis Rheum. 52 (4): 1264–73. DOI:10.1002/art.20983. PMID 15818684. Research Blogging.
- ↑ Anonymous (2009). Milnacipran (Savella) for Fibromyalgia. The Medical Letter on Drugs and Therapeutics.
- ↑ Russell IJ, Perkins AT, Michalek JE (January 2009). "Sodium oxybate relieves pain and improves function in fibromyalgia syndrome: a randomized, double-blind, placebo-controlled, multicenter clinical trial". Arthritis Rheum. 60 (1): 299–309. DOI:10.1002/art.24142. PMID 19116896. Research Blogging.