Horizontal gene transfer/Citable Version: Difference between revisions
imported>David Tribe |
Pat Palmer (talk | contribs) |
||
| (381 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | |||
[[Image:Legionella pneumophila-s.jpg|right|frame|''[[Legionella pneumophila]]'' are [[prokaryote|prokaryotic]] bacteria that can survive and reproduce inside [[phagocytic]] cells such as protists that have eaten them. They are competent in DNA [[transformation]] and occasionally capture genes from their [[eukaryotic]] host cells.]] | |||
The | '''Horizontal gene transfer''' occurs when an [[organism]] transfers its genetic material to a being ''other'' than one of its own offspring. The actual process of this transfer can be by any mechanism, but because genes are not passing by descent, horizontal gene transfer (abbreviated as HGT) is always very different from [[Biological inheritance|vertical gene transfer]]. In vertical descent, parental traits are inherited by progeny by one of two general methods: either (1) ''sexual reproduction'' in which [[gametes]] form [[zygotes]], a common method in higher animals and plants, or (2) by ''asexual reproduction'', where splitting of cells or an entire organism grows from a fragment, as is usual in [[bacteria]] and [[Fungus|fungi]], but which also happens in some animals and plants. HGT is a much more recently discovered route of passage for genetic material; it is relatively common in [[microorganism]]s, and to a lesser extent in plants. By HGT, genetic material can be shared between organisms without the immediate relatedness of mother cell to daughter cells, or parent organisms to offspring; indeed, by HGT material can pass between organismsthat are not even be of the same [[species]], [[genus]], sub-kingdom or kingdom of life form. HGT (sometimes called ''lateral'' gene transfer) is very much less common than vertical gene transfer, so its detection requires special techniques. | ||
==Introduction== | |||
, | Evidence from [[genomics|genome]] science and [[bioinformatics]] shows that HGT has occurred between diverse biological [[taxa]] that are widely separated in the [[phylogeny|phylogenetic]] [http://www.tolweb.org/tree/ tree of life]. Known HGTs include movement of genetic material between different species of microbes and other microbial taxa such as protists, gene movement between different plant families, between different animals, and between bacteria and plants. [[Image:Cobwebsoflife.jpg|right|frame|HGT — gene exchange between non-related organisms —appears commonplace among bacteria, but contributes just small fragments of genetic information, leaving the traditional tree of life intact. From: [http://biology.plosjournals.org/perlserv/?request=slideshow&type=figure&doi=10.1371/journal.pbio.0030347&id=36052 Comparing Gene Trees and Genome Trees: A Cobweb of Life? PLoS Biol 3:e347]]] | ||
HGT is | Microorganisms appear to be most affected by HGT, but even in microbes only about 2% of core genes are transferred laterally. Because this percentage is so low, the main lineages of microbial evolution can still be treated as 'trees' branched by vertical descent, with HGT included in the scheme only as 'cobwebs' (see figure at right). | ||
Gene transfers between different biological [[Three-domain system|sub-kingdoms]] (domains), such as between [[eukaryote|eukaryotic]] protists and bacteria, or between bacteria and insects are the most phylogenetically extreme cases of HGT. An example is bacterial 'rol' genes from ''Agrobacterium'' species which have been found in tobacco plants (''Nicotiniana'').<ref>de Felipe K ''et al.'' (2005) Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol [http://jb.asm.org/cgi/content/full/187/22/7716?view=long&pmid=16267296 187:7716-26] PMID 16267296 (Open access) | |||
* Kondo N ''et al.'' (2002) Genome fragment of ''Wolbachia'' endosymbiont transferred to X chromosome of host insect. Proc Natl Acad Sci USA [http://www.pnas.org/cgi/content/full/99/22/14280 99:14280-5] PMID 12386340 (Open access) | |||
* Intrieri MC, Buiatti M (2001) The horizontal transfer of ''Agrobacterium'' rhizogenes genes and the evolution of the genus ''Nicotiana''. Mol Phylogen Evol 20:100-10 PMID 11421651</ref> | |||
HGT is just one of several processes that can cause rearrangement of genomes during evolution. The possibility of intracellular movement of genes between different parts of an organism's genome (that is, between the [[chromosomes]] of the [[nucleus]], the circular [[mitochondrion]] chromosome, or the circular [[plastid]] ([[chloroplast]]) chromosome) needs to be considered when evaluating HGT between different species.<ref>Timmis JN ''et al.'' (2004) Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat Rev Genet 5:123-35 PMID 14735123</ref> | |||
==Main features of HGT in nature== | ==Main features of HGT in nature== | ||
* A hallmark of HGT is the presence of the same gene in organisms that are only very distantly related to each other. The frequent discovery of shared DNA sequences such as the ''mariner'' class of [[transposons]], [[insertion sequence]] DNA, [[retrovirus]] genes in diverse species and shared mitochondrial genes in diverse flowering plants indicate that [[mobile DNA]] has natural pathways for movement between species. (The name mariner for a class of related transposons is an allusion to ''The Rime of the Ancient Mariner'', meaning a traveller to distant lands.) Close relatives of ''mariner'' mobile DNA have been identified in organisms as diverse as mites, flatworms, hydras, insects, nematodes, mammals and humans.<ref>Robertson HM (1996) Reconstruction of the ancient ''mariners'' of humans. Nat Genet 12:360-1 PMID 8630486 | |||
*[http://etext.virginia.edu/toc/modeng/public/Col2Mar.html ''The Rime of the Ancient Mariner'']Samuel Taylor Coleridge(1772-1834)</ref> | |||
* | |||
[[Image:Millet.jpg|right|frame|Millet. From: [http://biology.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pbio.0040035 ''Jumping Genes Cross Plant Species Boundaries.'']Analysis of the genomes of millet and rice revealed evidence for HGT between chromosomes in the nucleus of one plant to chromosomes in the nucleus of a reproductively isolated species]] | |||
* | * Horizontal movement of genes is common among bacteria, and is a major factor in accelerating the rate of their evolution. HGT is involved in multiple-antibiotic resistance in pathogenic bacteria, and this is a major factor that is limiting the effectiveness of antibiotics. Inter-domain (sub-kingdom) transfer of several genes from eukaryotes to bacteria for instance, has occurred in the 'accidentally pathogenic' bacterium (''Legionella pneumophila'', see illustration) that lives within vacuoles of [[protist]] and mammalian [[macrophage]] cells.<ref>Jain R ''et al.'' (2003) Horizontal gene transfer accelerates genome innovation and evolution. Mol Biol Evol [http://mbe.oxfordjournals.org/cgi/content/full/20/10/1598 20:1598-602] PMID 12777514 (Open access) | ||
* de Felipe KS ''et al.'' (2005) Evidence for acquisition of ''Legionella'' type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol[http://jb.asm.org/cgi/content/full/187/22/7716?view=long&pmid=16267296 187:7716-26] PMID 16267296 (Open access) </ref> | |||
* '' | * HGT is documented in diverse unicellular protists, which can contain several genes transferred from both [[prokaryotes]] and other protists.<ref>Andersson JO ''et al.'' (2006) Evolution of four gene families with patchy phylogenetic distributions: influx of genes into protist genomes.[http://www.biomedcentral.com/1471-2148/6/27 BMC Evol Biol] PMID 16551352 (Open access) | ||
* Loftus B ''et al.'' (2005) The genome of the protist parasite ''Entamoeba histolytica''. Nature 433:865-8 PMID 15729342</ref> | |||
* Not all of | * HGT occurs globally on a massive scale among marine microorganisms. Viruses, which, at total numbers near 10<sup>29</sup> are the most common biological entities in the sea, are a major pathway for gene movement between different species. It has been estimated that, on average, 10<sup>13</sup> virus-mediated gene transfer events occur in the Mediterranean sea each year. [[Endosymbiosis]] with an alga is identified as a route for HGT in marine [[dinoflagellates]], the organisms that cause 'red tides'.<ref>Fuhrman JA (1999) Marine viruses and their biogeochemical and ecological effects. Nature 399:541–8 PMID 10376593 | ||
*Yoon HS ''et al.'' (2005) Tertiary endosymbiosis driven genome evolution in dinoflagellate algae. Mol Biol Evol [http://mbe.oxfordjournals.org/cgi/content/full/22/5/1299 22:1299-308] PMID 15746017 (Open access)</ref> | |||
* Interspecies gene movement by cross-hybridization is common in flowering plants. Mechanisms for HGT in flowering plants between more distant taxa involving parasitic plants such as dodder and endophytes (such as mosses, which are in intimate cell-to-cell contact with their host plants) are also well established (see [[Horizontal gene transfer in plants]]). Plant mitochondria can be unusually active in HGT. | |||
* Not all of the ways in which HGT occurs are fully characterized, but some have been identified. HGT is hard to detect directly, as it is relatively rare within a species, but can be detected by modern DNA analysis which can enable detailed comparison of [[genomics|genome]]s. In insects, mites and viruses are probably vectors for HGT. In certain bacteria, surface appendages called [[Pilus|pili]] have various roles in DNA uptake, DNA secretion and DNA transfer which have been extensively analyzed; HGT in bacteria includes [[plasmid]]-mediated promiscuous mating by bacteria (for instance by the crown-gall bacterium ''Agrobacterium tumefaciens'') and carriage of genes between species by viruses. Direct DNA uptake is another transfer mechanism, as illustrated by ''Legionella'' bacteria, which are naturally competent for DNA uptake. | |||
==Prokaryotes== | ==Prokaryotes== | ||
:''See main article [[Horizontal gene transfer in prokaryotes]]'' | :''See main article [[Horizontal gene transfer in prokaryotes|HGT in prokaryotes]]'' | ||
:''The three main mechanisms of HGT in | :''The three main mechanisms of HGT in bacteria and archaea discussed here are:'' | ||
:* '''''Bacterial [[Transformation (genetics)|Transformation]]''' or direct uptake of extracellular DNA.'' | :* '''''Bacterial [[Transformation (genetics)|Transformation]]''' or direct uptake of extracellular DNA.'' | ||
:* '''''[[Transduction (genetics)|Transduction]]''' of genes by bacterial viruses.'' | :* '''''[[Transduction (genetics)|Transduction]]''' of genes by bacterial viruses.'' | ||
:* '''''[[Bacterial conjugation]]''', a gene transfer process carried out by | :* '''''[[Bacterial conjugation]]''', a gene transfer process carried out by plasmids and conjugative transposons.'' | ||
==Eukaryotes== | ==Eukaryotes== | ||
: ''See also [[Endosymbiotic theory]]'' | |||
===Protists=== | ===Protists=== | ||
Analysis of the complete genome sequence of the protist ''Entamoeba histolytica'' indicates 96 cases of relatively recent | Analysis of the complete genome sequence of the protist ''Entamoeba histolytica'' indicates 96 cases of relatively recent HGT from prokaryotes. There is also convincing evidence that a bacterial gene for a biosynthetic enzyme has been recruited by the protist ''Trichomonas vaginalis'' from bacteria related to the ancestors of ''Pasteurella'' bacteria. Similar analysis of the protist ''Cryptosporidium parvum'' reveals 24 candidates of HGT from bacteria. These results fit the idea that 'you are what you eat'. That is, in unicellular grazing organisms, foreign genetic material is constantly entering the cell from food organisms, and occasionally some of this material enters the genome.<ref>Loftus B ''et al.'' (2005) The genome of the protist parasite ''Entamoeba histolytica''. Nature 433:865-8 PMID 15729342 | ||
* Doolittle WF (1998) You are what you eat: a gene transfer ratchet could account for bacterial genes in eukaryotic nuclear genomes. Trends in Genetics 14:307-11 PMID 9724962</ref> | |||
===Fungi=== | ===Fungi=== | ||
Comparison of the genome sequences of two fungi, | Comparison of the genome sequences of two fungi, baker's yeast (''Saccharomyces cerevisiae'') and ''Ashbya gossypii'', has shown that ''Saccharomyces'' has received two genes from bacteria by HGT. One of these genes codes for an enzyme that allows baker's yeast to make pyrimidine nucleotide bases anaerobically, and the other allows usage of sulfur from several organic sulfur sources. Other work with yeasts suggests that eight genes from ''Yarrowia lipolytica'', five from ''Kluyveromyces lactis'', and one from ''Debaryomyces hansenii'' are horizontally transferred.<ref>Dujon B ''et al.'' {2004) Genome evolution in yeasts. Nature 430:35-44 PMID 15229592</ref> | ||
===Other eukaryotes=== | ===Other eukaryotes=== | ||
Analysis of [[DNA sequence]]s suggests that HGT has also | Analysis of [[DNA sequence]]s suggests that HGT has also occurred within multicellular eukaryotes, by a route that involves transfer of genes from chloroplast and mitochondrial genomes to the nuclear genomes.<ref>Adams KL Palmer JD (2003) Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol Phylogenet Evol 29:380–95 PMID 14615181</ref> According to the [[endosymbiotic theory]], chloroplasts and mitochondria originated as the bacterial [[endosymbiont]]s of a progenitor to the eukaryotic cell, and endosymbiosis can be considered to be a special case of HGT. | ||
===Plants=== | ===Plants=== | ||
| Line 56: | Line 60: | ||
:''See [[Transgenic plant]] for hybridization by cross-pollination and artificial horizontal gene transfer in [[biotechnology]].'' | :''See [[Transgenic plant]] for hybridization by cross-pollination and artificial horizontal gene transfer in [[biotechnology]].'' | ||
It has also been discovered that plant genes can move to endophyte fungi that grow on them. Several plant endophyte fungi that grow on taxol-producing yew trees have gained the ability to make taxol themselves.<ref>Shrestha K ''et al.'' (2001) Evidence for paclitaxel from three new endophytic fungi of Himalayan yew of Nepal. Planta Med 67:374-6 PMID 11458463</ref> (Taxol, also called paclitaxel, is an anti-cancer drug found in yew trees.) | |||
===Animals=== | ===Animals=== | ||
[[Junk DNA]] is the most obvious general evidence of HGT in eukaryotes. | [[Junk DNA]] is the most obvious general evidence of HGT in eukaryotes. Junk DNA is the name given to the seemingly non-functional repetitive DNA sequences that are a major portion of the genomes of many plants and animals. This DNA usually includes multiple copies of various '[[Jumping genes]]' which can proliferate within a genome after they have been transferred from another species. Examples in the human of such mobile elements are 'Hsmar1' and 'Hsmar2' which are related to the widely studied 'mariner' transposon. Close relatives of mariner mobile DNA have been discovered in organisms as diverse as mites, flatworms, hydras, insects, nematodes, mammals and humans.<ref>Robertson HM ''et al.'' (1996) Reconstruction of the ancient 'mariners' of humans. Nat Genet 12:360-361 PMID 8630486</ref> [[Retroviruses]] and [[retrotransposons]] are other examples of mobile horizontally transferred DNA found in animals. | ||
The adzuki bean beetle | The adzuki bean beetle ''Callosobruchus chinensis'' is infected with several strains of bacterial ''Wolbachia'' [[endosymbiont]]s. A genome fragment of one of these endosymbionts has been found transferred to the X chromosome of the host insect.<ref>Kondo N ''et al.'' (2002)Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect.Proc Natl Acad Sci USA [http://www.pnas.org/cgi/content/full/99/22/14280 99:14280-5] PMID 12386340 (Open access)</ref> | ||
==History of discovery of HGT== | ==History of discovery of HGT== | ||
:: ''See main article [[Horizontal gene transfer (History)]]'' | :: ''See main article [[Horizontal gene transfer (History)]]'' | ||
:*''Bacterial genetics starts in 1946'' | :*''Bacterial genetics starts in 1946'' | ||
:: ''see | :: ''see main article [[Horizontal gene transfer in prokaryotes]] | ||
:*''First glimpses of horizontal transfer of traits in plant evolution'' | :*''First glimpses of horizontal transfer of traits in plant evolution'' | ||
:: '' see also main article [[Barbara McClintock]] | :: '' see also main article [[Barbara McClintock]] | ||
| Line 72: | Line 76: | ||
:*''HGT and genetic engineering'' | :*''HGT and genetic engineering'' | ||
== | ==Decoding the tree of life from genomes scrambled by HGT== | ||
: ''For more information, see Citizendium's article on [[Prokaryote phylogeny and evolution]]'' | |||
HGT | |||
'' | |||
Because each organism carries a record of its ancestry in its DNA, methods for rapid gene isolation and analysis of DNA encoded sequences - which can extract the information from this ancestral archive - have been important for answering questions about evolution, and they have enabled rapid expansion of the field of biological [[systematics]] known as [[Molecular phylogeny|phylogenetic inference]]. Comparison of related ([[homologous]]) gene sequences from different organisms has made it possible to reconstruct the history of many evolutionary lineages.<ref>Steenkamp ET ''et al.'' (2006) The protistan origins of animals and fungi. Mol Biol Evol [http://mbe.oxfordjournals.org/cgi/content/full/23/1/93 23:93-106] PMID 16151185 (Open access)</ref> | |||
Many issues about evolution have been clarifed by comparing homologous genes from different species, genera, families, and phyla. Unfortunately, HGT complicates the picture, because HGT 'scrambles' the evidence needed to deduce the branching patterns of evolutionary trees. One area of current research in phylogenetic inference, and arguably one of the most challenging problems in evolutionary theory, is the early stages in the [http://tolweb.org/Life_on_Earth/1 evolution of life]. This quandary about the origins of different cell types provides a good illustration of the complications introduced by HGT into the reconstruction of evolutionary history. | |||
The main early branches of the tree of life have been intensively studied by [[Microorganism|microbiologists]] because the first organisms were microrganisms. A gene very often used for constructing phylogenetic relationships in microorganisms is the small ribosomal subunit ribosomal RNA (SSU rRNA) gene, as its sequences tend to be conserved among members with close phylogenetic distances, yet it is variable enough that differences can be measured.<ref>Woese C ''et al.'' (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA [http://www.pnas.org/cgi/reprint/87/12/4576 87:4576-9] PMID 2112744 (Open access) | |||
< | *Woese C, Fox G (1977). Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci USA [http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=270744 74:5088-90] PMID 270744</ref> The use of SSU rRNA to measure evolutionary "distances" was pioneered by [[Carl Woese]] when formulating the first modern 'tree of life', and his results led him to propose the [[Archaea]] (single celled organisms superficially similar to bacteria) as a third domain (sub-kingdom) of life. | ||
[[Image:Tree_phylogeny_3_domain.gif|thumb|300px|left|A three domain representation of the [[phylogenetic tree|tree]] of life based on SSU rRNA sequences, showing the separation of Bacteria, Archaea, and Eukaryote domains. See [[Microorganisms]] article for further explanation]] | |||
Microbiologists introduced the term ''domain'' for the three main early branches of the tree of life, where ''domain'' is a [[phylogenetic]] term very similar in meaning to biological kingdom. These represent the three main lineages in evolution of early cellular life, and are currently represented by the ''Bacteria'', the ''Archaea'' and ''Eukarya (eukaryote)'' domains. Eukaryotes are all organisms with a well defined nucleus, and this domain comprises protists, fungi, and all organisms in the animal and plant kingdoms, including humans. As seen in the figure (left), studies of SSU rRNA genes (and some other genes) might suggest that ''Archaea'' and ''Eukarya'' have a ''sister'' relationship in evolution, and it has often been assumed that eukaryotes evolved from archaeal cells. However, the fact that genes can move between distant branches of the tree of life even at low probabilities poses problems for scientists trying to reconstruct evolution from studying genes and gene sequences in different organisms due to the scrambling effect of HGT. The challenges are most awkward for the ambitious reconstruction of the earliest branches of the tree of life - because over a long enough time and with large numbers of organisms, many HGT events are certain to have occurred even though each particular transfer event has a low probability. | |||
Thus recent discoveries of 'rampant' HGT in microorganisms, and the detection of horizontal movement of genes for SSU rRNA have forced biologists to question the accuracy of at least the early branches in the tree of life, and even to question the validity of trees as useful models of microbial evolution.<ref>Simonson AB ''et al.'' (2005) Decoding the genomic tree of life. Proc Natl Acad Sci USA[http://www.pnas.org/cgi/content/full/102/suppl_1/6608 102 Suppl 1:6608-13] PMID 15851667 (Open access) | |||
*Yap WH ''et al.'' (1999) Distinct types of rRNA operons exist in the genome of the actinomycete ''Thermomonspora chromogena'' and evidence for horizontal gene transfer of an entire rRNA operon. J Bacteriol [http://jb.asm.org/cgi/content/full/181/17/5201?view=long&pmid=10464188 181:5201-9] PMID 10464188 (Open access) | |||
* Gogarten JP Townsend JP (2005) Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol 9:679-87 PMID 16138096</ref> | |||
=== | ===Recent efforts to infer evolutionary trees while recognizing HGT=== | ||
: ''For more information, see Citizendium's article on [[Evolution of cells]]'' | |||
The challenges of ascertaining an accurate branching structure for evolutionary trees of microbes, especially the earliest branches in the tree of life, have been a great spur for current biological research. Clear answers to important questions about these trees are not yet available, but many interesting discoveries and ideas are emerging. | |||
Instead of relying primarily on a single gene such as the SSU rRNA gene to reconstruct evolution, scientific effort has now shifted to exploiting the comprehensive information from the many complete genome sequences of organisms that are now available.<ref>Eisen JA, Fraser CM (2003) Viewpoint phylogenomics: intersection of evolution and genomics. Science 300:1706-7 PMID 12805538 | |||
* Fitzpatrick DA ''et al.'' (2006) A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol Biol[http://www.biomedcentral.com/1471-2148/6/99 6:99] PMID 17121679 (Open access) | |||
* Ge F ''et al.'' (2005) The Cobweb of Life revealed by genome-scale estimates of horizontal gene transfer. PLoS Biol[http://biology.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pbio.0030316 3:e316] PMID 16122348 (Open access) | |||
* Henz SR ''et al.'' (2005) Whole-genome prokaryotic phylogeny.Bioinformatics [http://bioinformatics.oxfordjournals.org/cgi/content/full/21/10/2329 21:2329-35] PMID 15166018 (Open access) | |||
* Urwin R, Maiden MC (2003) Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol 11:479-87 PMID 14557031</ref> So far, this comparative [[genomics]] approach, made possible by specially developed computer programs and mathematical algorithms, suggests that most of the core genes of bacteria that are useful for deducing evolutionary histories are unaffected by HGT. This confirms the practical experience of microbiologists that consistent and reliable trees can still be deduced for the more recent stages in microbial evolution, such as evolutionary relationships within particular bacterial phyla. This does require, however, using multiple, well chosen genes to investigate how lineages are related to one another. Thus the 'tree' is still a valid metaphor for microbial evolution - but a tree adorned with 'cobwebs' of horizontally transferred genes. | |||
But it is also clear that trees based only on SSU rRNA alone do not capture the events of early eukaryote evolution accurately, and the origins of the first nucleated cells are still uncertain. For instance, careful analysis of the complete genome of the eukaryote yeast shows that many of its genes are more closely related to bacterial genes than they are to archaea, and it is now clear that archaea were not the simple progenitors of the eukaryotes. This discovery is a stark contradiction to earlier findings based on SSU rRNA and limited samples of other genes.<ref>Esser C ''et al.'' (2004) A genome phylogeny for mitochondria among alpha-proteobacteria and a predominantly eubacterial ancestry of yeast nuclear genes. Mol Biol Evol [http://mbe.oxfordjournals.org/cgi/content/full/21/9/1643 21:1643-50] PMID 15155797 (Open access)</ref> (See [[Evolution of cells]] for further discussion.) | |||
[[ | On the other hand, the concept that rampant HGT took place in 'gene-swapping collectives' involved in metabolism and replication at the [[Origin of life|earliest stages of life's origins]], before a postulated transition to Darwinian evolution of the cellular lineages known today, has been used by Carl Woese and colleagues to develop fresh insight into the origins of the universal [[genetic code]].<ref>Goldenfeld N, Woese C (2007) Essays: Connections. Biology's next revolution The emerging picture of microbes as gene–swapping collectives demands a revision of such concepts as organism, species and evolution itself. Nature 445:369 [http://nature.com/nature/focus/arts/connections/index.html doi:10.1038/445369a] | ||
* Vetsigian K ''et al.'' (2006) Collective evolution and the genetic code. Proc Natl Acad Sci USA [http://www.pnas.org/cgi/content/full/103/28/10696 103:10696–700] PMID 1681888</ref> | |||
==See also== | ==See also== | ||
*[[Gene flow]] | *[[Gene flow]] | ||
*[[Species]] | |||
*[[Phylogenetic tree]] | *[[Phylogenetic tree]] | ||
*[[ | *[[Evolution of cells]] | ||
*[[Germline]] | *[[Germline]] | ||
*[[Systems biology]] | |||
*[[Origin of life]] | |||
*[[Mitochondrion]] | *[[Mitochondrion]] | ||
*[[Endosymbiont]] | |||
*[[Endosymbiotic theory]] | |||
*[[Integron]] | *[[Integron]] | ||
*[[Virus]] | |||
*[[Provirus]] | *[[Provirus]] | ||
*[[Retrotransposon]] | *[[Retrotransposon]] | ||
*[[Endogenous retrovirus]] | |||
*[[Plasmid]] | |||
*[[Mobile DNA]] | *[[Mobile DNA]] | ||
*[[Pilus]] | *[[Pilus]] | ||
*[[Transgenic plant]] | |||
==References== | ==References== | ||
===Citations=== | ====Citations==== | ||
<references/> | <div class="references-small" style="-moz-column-count:2; column-count:2;"> | ||
<references /> | |||
</div> | |||
===Further | ====Further reading==== | ||
* | *[http://www.nature.com/nrmicro/focus/genetransfer/index.html Focus on horizontal gene transfer] Webfocus in ''Nature'' with free access review articles. | ||
*[http://cryptome.org/smallpox-wmd.htm Smallpox knows how to make a mouse protein. How did smallpox learn that?] ''The New Yorker'' July 12, 1999, p44-61. 'The poxviruses are promiscuous at capturing genes from their hosts,' Esposito said. 'It tells you that smallpox was once inside a mouse or some other small rodent'. (Open access) | |||
* | * ''[http://biology.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pbio.0030169 Where Do All Those Genes Come From?]'' This study resolves a long-standing paradox: how is it possible to deduce reliable evolutionary histories from gene sequences in bacteria despite extensive HGT? (Open access) | ||
* Woese C (2002) [http://www.pnas.org/cgi/content/full/99/13/8742 On the evolution of cells.]Proc Natl Acad Sci USA 99:8742-7 PMID 12077305. This article shifts the emphasis in early phylogenic adaptation from vertical to horizontal gene transfer. (Open access) | |||
* | * Salzberg SL ''et al.'' (2001) [http://www.cbcb.umd.edu/~salzberg/docs/ScienceLateralTransfer.pdf Microbial genes in the human genome: lateral transfer or gene loss?] Science 292:1903-6 PMID 11358996. This reports that one dramatic claim of HGT - in which a distinguished group of scientists claimed that bacteria transferred their DNA directly into the human lineage - was simply wrong. (Open access) | ||
* Jain R ''et al.'' Horizontal gene transfer among genomes: the complexity hypothesis. Proc Natl Acad Sci USA [http://www.pnas.org/cgi/content/abstract/96/7/3801 96:3801-6] PMID 10097118 (Open access) | |||
* | * Hall C ''et al.'' (2005) Contribution of horizontal gene transfer to the evolution of ''Saccharomyces cerevisiae''. Eukaryot Cell [http://ec.asm.org/cgi/content/full/4/6/1102 4:1102-15] PMID 15947202 Convincing evidence of horizontal transfer of bacterial DNA into yeast. (Open access.) | ||
* Zhu J ''et al.'' (2000) The bases of crown gall tumorigenesis.J Bacteriol [http://jb.asm.org/cgi/content/full/182/14/3885?view=long&pmid=10869063 182:3885-95] PMID 10869063 This article describes the biology of crown-gall bacterium, and the mechanism of DNA injection by this bacterium, and explains how genes can move between bacterial species and from bacteria to eukaryotic organisms, and illustrates the extent to which different species can co-evolve. (Open access) | |||
* '' | * ''Horizontal Gene Transfer'' Syvanen M, Kado CI (2002) 2nd edition, Academic Press ISBN 0-12-680126-6 A comprehensive treatise. [http://www.nature.com/hdy/journal/v90/n1/full/6800196a.html Reviewed here by M-W Ho] | ||
* | * ''Acquiring genomes: a theory of the origin of species.'' Margulis L and Sagan D (2002) Basic Books ISBN 0-465-04392-5. A book that looks at gene transfer from a different perspective to many conventional interpretations, but with an emphasis on microbial diversity. [http://home.planet.nl/~gkorthof/korthof72.htm Reviewed here.] | ||
* Richardson AO, Palmer, JD (2007) Horizontal gene transfer in plants. J Exp Bot 58:1–9 doi:10.1093/jxb/erl148 PMID 17030541 | |||
* Gogarten JP Townsend JP (2005) Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol 9:679-87 PMID 16138096. One article in a whole issue of the journal ''Nature Reviews Microbiology'' largely devoted to HGT. | |||
* Weinbauer MG, Rassoulzadegan F (2004) Are viruses driving microbial diversification and diversity? Envir Microbiol [http://www.blackwell-synergy.com/links/doi/10.1046/j.1462-2920.2003.00539.x/full/ 6:1-11 ] PMID 14686936 Discussion of both the evolutionary and ecological activities of viruses in the ocean, a major source of HGT in nature. | |||
===External links=== | ====External links==== | ||
* | * [http://opbs.okstate.edu/~melcher/MG/MGW3/MG334.html Horizontal gene transfer] (p334 of Molecular Genetics by Ulrich Melcher). | ||
*[http://www. | *[http://www.sci.sdsu.edu/~smaloy/MicrobialGenetics/topics/genetic-exchange/exchange/exchange.html Horizontal gene transfer at sciences.sdsu.edu] | ||
*[http://www.stat.rice.edu/~mathbio/Ochman2000.pdf | *[http://www.stat.rice.edu/~mathbio/Ochman2000.pdf Lateral gene transfer and the nature of bacterial innovation (pdf), Ochman ''et al.'' (2000)] | ||
* [http://gogarten.uconn.edu/ Gogarten Laboratory Webpages.] | |||
*[http:// | *[http://www.esalenctr.org/display/confpage.cfm?confid=10&pageid=105&pgtype=1 Horizontal gene transfer - A new paradigm for biology] | ||
*[http://www.esalenctr.org/display/confpage.cfm?confid=10&pageid=105&pgtype=1 Horizontal | *[http://www.i-sis.org.uk/ireaff99.php Report on horizontal gene transfer] by Mae-Wan Ho, 1999 | ||
*[http://www.i-sis.org.uk/FSAopenmeeting.php Recent evidence confirms risks of horizontal gene transfer] | |||
*[http://www.i-sis.org.uk/ireaff99.php Report on horizontal gene transfer by Mae-Wan Ho | |||
*[http://www.i-sis.org.uk/FSAopenmeeting.php Recent | |||
<br/> | <br/> | ||
Latest revision as of 09:01, 21 June 2024
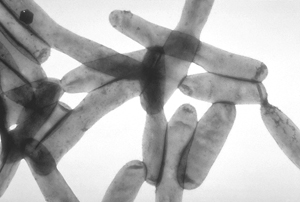
Horizontal gene transfer occurs when an organism transfers its genetic material to a being other than one of its own offspring. The actual process of this transfer can be by any mechanism, but because genes are not passing by descent, horizontal gene transfer (abbreviated as HGT) is always very different from vertical gene transfer. In vertical descent, parental traits are inherited by progeny by one of two general methods: either (1) sexual reproduction in which gametes form zygotes, a common method in higher animals and plants, or (2) by asexual reproduction, where splitting of cells or an entire organism grows from a fragment, as is usual in bacteria and fungi, but which also happens in some animals and plants. HGT is a much more recently discovered route of passage for genetic material; it is relatively common in microorganisms, and to a lesser extent in plants. By HGT, genetic material can be shared between organisms without the immediate relatedness of mother cell to daughter cells, or parent organisms to offspring; indeed, by HGT material can pass between organismsthat are not even be of the same species, genus, sub-kingdom or kingdom of life form. HGT (sometimes called lateral gene transfer) is very much less common than vertical gene transfer, so its detection requires special techniques.
Introduction
Evidence from genome science and bioinformatics shows that HGT has occurred between diverse biological taxa that are widely separated in the phylogenetic tree of life. Known HGTs include movement of genetic material between different species of microbes and other microbial taxa such as protists, gene movement between different plant families, between different animals, and between bacteria and plants.
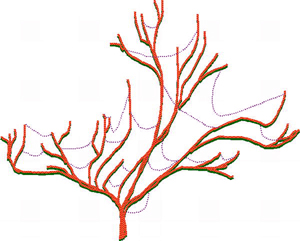
Microorganisms appear to be most affected by HGT, but even in microbes only about 2% of core genes are transferred laterally. Because this percentage is so low, the main lineages of microbial evolution can still be treated as 'trees' branched by vertical descent, with HGT included in the scheme only as 'cobwebs' (see figure at right).
Gene transfers between different biological sub-kingdoms (domains), such as between eukaryotic protists and bacteria, or between bacteria and insects are the most phylogenetically extreme cases of HGT. An example is bacterial 'rol' genes from Agrobacterium species which have been found in tobacco plants (Nicotiniana).[1]
HGT is just one of several processes that can cause rearrangement of genomes during evolution. The possibility of intracellular movement of genes between different parts of an organism's genome (that is, between the chromosomes of the nucleus, the circular mitochondrion chromosome, or the circular plastid (chloroplast) chromosome) needs to be considered when evaluating HGT between different species.[2]
Main features of HGT in nature
- A hallmark of HGT is the presence of the same gene in organisms that are only very distantly related to each other. The frequent discovery of shared DNA sequences such as the mariner class of transposons, insertion sequence DNA, retrovirus genes in diverse species and shared mitochondrial genes in diverse flowering plants indicate that mobile DNA has natural pathways for movement between species. (The name mariner for a class of related transposons is an allusion to The Rime of the Ancient Mariner, meaning a traveller to distant lands.) Close relatives of mariner mobile DNA have been identified in organisms as diverse as mites, flatworms, hydras, insects, nematodes, mammals and humans.[3]

- Horizontal movement of genes is common among bacteria, and is a major factor in accelerating the rate of their evolution. HGT is involved in multiple-antibiotic resistance in pathogenic bacteria, and this is a major factor that is limiting the effectiveness of antibiotics. Inter-domain (sub-kingdom) transfer of several genes from eukaryotes to bacteria for instance, has occurred in the 'accidentally pathogenic' bacterium (Legionella pneumophila, see illustration) that lives within vacuoles of protist and mammalian macrophage cells.[4]
- HGT is documented in diverse unicellular protists, which can contain several genes transferred from both prokaryotes and other protists.[5]
- HGT occurs globally on a massive scale among marine microorganisms. Viruses, which, at total numbers near 1029 are the most common biological entities in the sea, are a major pathway for gene movement between different species. It has been estimated that, on average, 1013 virus-mediated gene transfer events occur in the Mediterranean sea each year. Endosymbiosis with an alga is identified as a route for HGT in marine dinoflagellates, the organisms that cause 'red tides'.[6]
- Interspecies gene movement by cross-hybridization is common in flowering plants. Mechanisms for HGT in flowering plants between more distant taxa involving parasitic plants such as dodder and endophytes (such as mosses, which are in intimate cell-to-cell contact with their host plants) are also well established (see Horizontal gene transfer in plants). Plant mitochondria can be unusually active in HGT.
- Not all of the ways in which HGT occurs are fully characterized, but some have been identified. HGT is hard to detect directly, as it is relatively rare within a species, but can be detected by modern DNA analysis which can enable detailed comparison of genomes. In insects, mites and viruses are probably vectors for HGT. In certain bacteria, surface appendages called pili have various roles in DNA uptake, DNA secretion and DNA transfer which have been extensively analyzed; HGT in bacteria includes plasmid-mediated promiscuous mating by bacteria (for instance by the crown-gall bacterium Agrobacterium tumefaciens) and carriage of genes between species by viruses. Direct DNA uptake is another transfer mechanism, as illustrated by Legionella bacteria, which are naturally competent for DNA uptake.
Prokaryotes
- See main article HGT in prokaryotes
- The three main mechanisms of HGT in bacteria and archaea discussed here are:
- Bacterial Transformation or direct uptake of extracellular DNA.
- Transduction of genes by bacterial viruses.
- Bacterial conjugation, a gene transfer process carried out by plasmids and conjugative transposons.
Eukaryotes
- See also Endosymbiotic theory
Protists
Analysis of the complete genome sequence of the protist Entamoeba histolytica indicates 96 cases of relatively recent HGT from prokaryotes. There is also convincing evidence that a bacterial gene for a biosynthetic enzyme has been recruited by the protist Trichomonas vaginalis from bacteria related to the ancestors of Pasteurella bacteria. Similar analysis of the protist Cryptosporidium parvum reveals 24 candidates of HGT from bacteria. These results fit the idea that 'you are what you eat'. That is, in unicellular grazing organisms, foreign genetic material is constantly entering the cell from food organisms, and occasionally some of this material enters the genome.[7]
Fungi
Comparison of the genome sequences of two fungi, baker's yeast (Saccharomyces cerevisiae) and Ashbya gossypii, has shown that Saccharomyces has received two genes from bacteria by HGT. One of these genes codes for an enzyme that allows baker's yeast to make pyrimidine nucleotide bases anaerobically, and the other allows usage of sulfur from several organic sulfur sources. Other work with yeasts suggests that eight genes from Yarrowia lipolytica, five from Kluyveromyces lactis, and one from Debaryomyces hansenii are horizontally transferred.[8]
Other eukaryotes
Analysis of DNA sequences suggests that HGT has also occurred within multicellular eukaryotes, by a route that involves transfer of genes from chloroplast and mitochondrial genomes to the nuclear genomes.[9] According to the endosymbiotic theory, chloroplasts and mitochondria originated as the bacterial endosymbionts of a progenitor to the eukaryotic cell, and endosymbiosis can be considered to be a special case of HGT.
Plants
- See Horizontal gene transfer in plants for
- Natural gene transfer between plants that do not cross-pollinate
- Jumping genes cross naturally between rice and millet
- Epiphytes and parasites as a bridge for gene flow between diverse plant species
- See Transgenic plant for hybridization by cross-pollination and artificial horizontal gene transfer in biotechnology.
It has also been discovered that plant genes can move to endophyte fungi that grow on them. Several plant endophyte fungi that grow on taxol-producing yew trees have gained the ability to make taxol themselves.[10] (Taxol, also called paclitaxel, is an anti-cancer drug found in yew trees.)
Animals
Junk DNA is the most obvious general evidence of HGT in eukaryotes. Junk DNA is the name given to the seemingly non-functional repetitive DNA sequences that are a major portion of the genomes of many plants and animals. This DNA usually includes multiple copies of various 'Jumping genes' which can proliferate within a genome after they have been transferred from another species. Examples in the human of such mobile elements are 'Hsmar1' and 'Hsmar2' which are related to the widely studied 'mariner' transposon. Close relatives of mariner mobile DNA have been discovered in organisms as diverse as mites, flatworms, hydras, insects, nematodes, mammals and humans.[11] Retroviruses and retrotransposons are other examples of mobile horizontally transferred DNA found in animals.
The adzuki bean beetle Callosobruchus chinensis is infected with several strains of bacterial Wolbachia endosymbionts. A genome fragment of one of these endosymbionts has been found transferred to the X chromosome of the host insect.[12]
History of discovery of HGT
- See main article Horizontal gene transfer (History)
- Bacterial genetics starts in 1946
- see main article Horizontal gene transfer in prokaryotes
- First glimpses of horizontal transfer of traits in plant evolution
- see also main article Barbara McClintock
- Discovery of mobile genes in flies, and mariner
- HGT and genetic engineering
Decoding the tree of life from genomes scrambled by HGT
- For more information, see Citizendium's article on Prokaryote phylogeny and evolution
Because each organism carries a record of its ancestry in its DNA, methods for rapid gene isolation and analysis of DNA encoded sequences - which can extract the information from this ancestral archive - have been important for answering questions about evolution, and they have enabled rapid expansion of the field of biological systematics known as phylogenetic inference. Comparison of related (homologous) gene sequences from different organisms has made it possible to reconstruct the history of many evolutionary lineages.[13] Many issues about evolution have been clarifed by comparing homologous genes from different species, genera, families, and phyla. Unfortunately, HGT complicates the picture, because HGT 'scrambles' the evidence needed to deduce the branching patterns of evolutionary trees. One area of current research in phylogenetic inference, and arguably one of the most challenging problems in evolutionary theory, is the early stages in the evolution of life. This quandary about the origins of different cell types provides a good illustration of the complications introduced by HGT into the reconstruction of evolutionary history.
The main early branches of the tree of life have been intensively studied by microbiologists because the first organisms were microrganisms. A gene very often used for constructing phylogenetic relationships in microorganisms is the small ribosomal subunit ribosomal RNA (SSU rRNA) gene, as its sequences tend to be conserved among members with close phylogenetic distances, yet it is variable enough that differences can be measured.[14] The use of SSU rRNA to measure evolutionary "distances" was pioneered by Carl Woese when formulating the first modern 'tree of life', and his results led him to propose the Archaea (single celled organisms superficially similar to bacteria) as a third domain (sub-kingdom) of life.
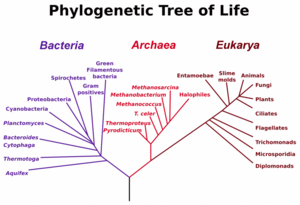
Microbiologists introduced the term domain for the three main early branches of the tree of life, where domain is a phylogenetic term very similar in meaning to biological kingdom. These represent the three main lineages in evolution of early cellular life, and are currently represented by the Bacteria, the Archaea and Eukarya (eukaryote) domains. Eukaryotes are all organisms with a well defined nucleus, and this domain comprises protists, fungi, and all organisms in the animal and plant kingdoms, including humans. As seen in the figure (left), studies of SSU rRNA genes (and some other genes) might suggest that Archaea and Eukarya have a sister relationship in evolution, and it has often been assumed that eukaryotes evolved from archaeal cells. However, the fact that genes can move between distant branches of the tree of life even at low probabilities poses problems for scientists trying to reconstruct evolution from studying genes and gene sequences in different organisms due to the scrambling effect of HGT. The challenges are most awkward for the ambitious reconstruction of the earliest branches of the tree of life - because over a long enough time and with large numbers of organisms, many HGT events are certain to have occurred even though each particular transfer event has a low probability.
Thus recent discoveries of 'rampant' HGT in microorganisms, and the detection of horizontal movement of genes for SSU rRNA have forced biologists to question the accuracy of at least the early branches in the tree of life, and even to question the validity of trees as useful models of microbial evolution.[15]
Recent efforts to infer evolutionary trees while recognizing HGT
- For more information, see Citizendium's article on Evolution of cells
The challenges of ascertaining an accurate branching structure for evolutionary trees of microbes, especially the earliest branches in the tree of life, have been a great spur for current biological research. Clear answers to important questions about these trees are not yet available, but many interesting discoveries and ideas are emerging.
Instead of relying primarily on a single gene such as the SSU rRNA gene to reconstruct evolution, scientific effort has now shifted to exploiting the comprehensive information from the many complete genome sequences of organisms that are now available.[16] So far, this comparative genomics approach, made possible by specially developed computer programs and mathematical algorithms, suggests that most of the core genes of bacteria that are useful for deducing evolutionary histories are unaffected by HGT. This confirms the practical experience of microbiologists that consistent and reliable trees can still be deduced for the more recent stages in microbial evolution, such as evolutionary relationships within particular bacterial phyla. This does require, however, using multiple, well chosen genes to investigate how lineages are related to one another. Thus the 'tree' is still a valid metaphor for microbial evolution - but a tree adorned with 'cobwebs' of horizontally transferred genes.
But it is also clear that trees based only on SSU rRNA alone do not capture the events of early eukaryote evolution accurately, and the origins of the first nucleated cells are still uncertain. For instance, careful analysis of the complete genome of the eukaryote yeast shows that many of its genes are more closely related to bacterial genes than they are to archaea, and it is now clear that archaea were not the simple progenitors of the eukaryotes. This discovery is a stark contradiction to earlier findings based on SSU rRNA and limited samples of other genes.[17] (See Evolution of cells for further discussion.)
On the other hand, the concept that rampant HGT took place in 'gene-swapping collectives' involved in metabolism and replication at the earliest stages of life's origins, before a postulated transition to Darwinian evolution of the cellular lineages known today, has been used by Carl Woese and colleagues to develop fresh insight into the origins of the universal genetic code.[18]
See also
- Gene flow
- Species
- Phylogenetic tree
- Evolution of cells
- Germline
- Systems biology
- Origin of life
- Mitochondrion
- Endosymbiont
- Endosymbiotic theory
- Integron
- Virus
- Provirus
- Retrotransposon
- Endogenous retrovirus
- Plasmid
- Mobile DNA
- Pilus
- Transgenic plant
References
Citations
- ↑ de Felipe K et al. (2005) Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol 187:7716-26 PMID 16267296 (Open access)
- Kondo N et al. (2002) Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect. Proc Natl Acad Sci USA 99:14280-5 PMID 12386340 (Open access)
- Intrieri MC, Buiatti M (2001) The horizontal transfer of Agrobacterium rhizogenes genes and the evolution of the genus Nicotiana. Mol Phylogen Evol 20:100-10 PMID 11421651
- ↑ Timmis JN et al. (2004) Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat Rev Genet 5:123-35 PMID 14735123
- ↑ Robertson HM (1996) Reconstruction of the ancient mariners of humans. Nat Genet 12:360-1 PMID 8630486
- The Rime of the Ancient MarinerSamuel Taylor Coleridge(1772-1834)
- ↑ Jain R et al. (2003) Horizontal gene transfer accelerates genome innovation and evolution. Mol Biol Evol 20:1598-602 PMID 12777514 (Open access)
- de Felipe KS et al. (2005) Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol187:7716-26 PMID 16267296 (Open access)
- ↑ Andersson JO et al. (2006) Evolution of four gene families with patchy phylogenetic distributions: influx of genes into protist genomes.BMC Evol Biol PMID 16551352 (Open access)
- Loftus B et al. (2005) The genome of the protist parasite Entamoeba histolytica. Nature 433:865-8 PMID 15729342
- ↑ Fuhrman JA (1999) Marine viruses and their biogeochemical and ecological effects. Nature 399:541–8 PMID 10376593
- Yoon HS et al. (2005) Tertiary endosymbiosis driven genome evolution in dinoflagellate algae. Mol Biol Evol 22:1299-308 PMID 15746017 (Open access)
- ↑ Loftus B et al. (2005) The genome of the protist parasite Entamoeba histolytica. Nature 433:865-8 PMID 15729342
- Doolittle WF (1998) You are what you eat: a gene transfer ratchet could account for bacterial genes in eukaryotic nuclear genomes. Trends in Genetics 14:307-11 PMID 9724962
- ↑ Dujon B et al. {2004) Genome evolution in yeasts. Nature 430:35-44 PMID 15229592
- ↑ Adams KL Palmer JD (2003) Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol Phylogenet Evol 29:380–95 PMID 14615181
- ↑ Shrestha K et al. (2001) Evidence for paclitaxel from three new endophytic fungi of Himalayan yew of Nepal. Planta Med 67:374-6 PMID 11458463
- ↑ Robertson HM et al. (1996) Reconstruction of the ancient 'mariners' of humans. Nat Genet 12:360-361 PMID 8630486
- ↑ Kondo N et al. (2002)Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect.Proc Natl Acad Sci USA 99:14280-5 PMID 12386340 (Open access)
- ↑ Steenkamp ET et al. (2006) The protistan origins of animals and fungi. Mol Biol Evol 23:93-106 PMID 16151185 (Open access)
- ↑ Woese C et al. (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 87:4576-9 PMID 2112744 (Open access)
- Woese C, Fox G (1977). Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci USA 74:5088-90 PMID 270744
- ↑ Simonson AB et al. (2005) Decoding the genomic tree of life. Proc Natl Acad Sci USA102 Suppl 1:6608-13 PMID 15851667 (Open access)
- Yap WH et al. (1999) Distinct types of rRNA operons exist in the genome of the actinomycete Thermomonspora chromogena and evidence for horizontal gene transfer of an entire rRNA operon. J Bacteriol 181:5201-9 PMID 10464188 (Open access)
- Gogarten JP Townsend JP (2005) Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol 9:679-87 PMID 16138096
- ↑ Eisen JA, Fraser CM (2003) Viewpoint phylogenomics: intersection of evolution and genomics. Science 300:1706-7 PMID 12805538
- Fitzpatrick DA et al. (2006) A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol Biol6:99 PMID 17121679 (Open access)
- Ge F et al. (2005) The Cobweb of Life revealed by genome-scale estimates of horizontal gene transfer. PLoS Biol3:e316 PMID 16122348 (Open access)
- Henz SR et al. (2005) Whole-genome prokaryotic phylogeny.Bioinformatics 21:2329-35 PMID 15166018 (Open access)
- Urwin R, Maiden MC (2003) Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol 11:479-87 PMID 14557031
- ↑ Esser C et al. (2004) A genome phylogeny for mitochondria among alpha-proteobacteria and a predominantly eubacterial ancestry of yeast nuclear genes. Mol Biol Evol 21:1643-50 PMID 15155797 (Open access)
- ↑ Goldenfeld N, Woese C (2007) Essays: Connections. Biology's next revolution The emerging picture of microbes as gene–swapping collectives demands a revision of such concepts as organism, species and evolution itself. Nature 445:369 doi:10.1038/445369a
- Vetsigian K et al. (2006) Collective evolution and the genetic code. Proc Natl Acad Sci USA 103:10696–700 PMID 1681888
Further reading
- Focus on horizontal gene transfer Webfocus in Nature with free access review articles.
- Smallpox knows how to make a mouse protein. How did smallpox learn that? The New Yorker July 12, 1999, p44-61. 'The poxviruses are promiscuous at capturing genes from their hosts,' Esposito said. 'It tells you that smallpox was once inside a mouse or some other small rodent'. (Open access)
- Where Do All Those Genes Come From? This study resolves a long-standing paradox: how is it possible to deduce reliable evolutionary histories from gene sequences in bacteria despite extensive HGT? (Open access)
- Woese C (2002) On the evolution of cells.Proc Natl Acad Sci USA 99:8742-7 PMID 12077305. This article shifts the emphasis in early phylogenic adaptation from vertical to horizontal gene transfer. (Open access)
- Salzberg SL et al. (2001) Microbial genes in the human genome: lateral transfer or gene loss? Science 292:1903-6 PMID 11358996. This reports that one dramatic claim of HGT - in which a distinguished group of scientists claimed that bacteria transferred their DNA directly into the human lineage - was simply wrong. (Open access)
- Jain R et al. Horizontal gene transfer among genomes: the complexity hypothesis. Proc Natl Acad Sci USA 96:3801-6 PMID 10097118 (Open access)
- Hall C et al. (2005) Contribution of horizontal gene transfer to the evolution of Saccharomyces cerevisiae. Eukaryot Cell 4:1102-15 PMID 15947202 Convincing evidence of horizontal transfer of bacterial DNA into yeast. (Open access.)
- Zhu J et al. (2000) The bases of crown gall tumorigenesis.J Bacteriol 182:3885-95 PMID 10869063 This article describes the biology of crown-gall bacterium, and the mechanism of DNA injection by this bacterium, and explains how genes can move between bacterial species and from bacteria to eukaryotic organisms, and illustrates the extent to which different species can co-evolve. (Open access)
- Horizontal Gene Transfer Syvanen M, Kado CI (2002) 2nd edition, Academic Press ISBN 0-12-680126-6 A comprehensive treatise. Reviewed here by M-W Ho
- Acquiring genomes: a theory of the origin of species. Margulis L and Sagan D (2002) Basic Books ISBN 0-465-04392-5. A book that looks at gene transfer from a different perspective to many conventional interpretations, but with an emphasis on microbial diversity. Reviewed here.
- Richardson AO, Palmer, JD (2007) Horizontal gene transfer in plants. J Exp Bot 58:1–9 doi:10.1093/jxb/erl148 PMID 17030541
- Gogarten JP Townsend JP (2005) Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol 9:679-87 PMID 16138096. One article in a whole issue of the journal Nature Reviews Microbiology largely devoted to HGT.
- Weinbauer MG, Rassoulzadegan F (2004) Are viruses driving microbial diversification and diversity? Envir Microbiol 6:1-11 PMID 14686936 Discussion of both the evolutionary and ecological activities of viruses in the ocean, a major source of HGT in nature.
External links
- Horizontal gene transfer (p334 of Molecular Genetics by Ulrich Melcher).
- Horizontal gene transfer at sciences.sdsu.edu
- Lateral gene transfer and the nature of bacterial innovation (pdf), Ochman et al. (2000)
- Gogarten Laboratory Webpages.
- Horizontal gene transfer - A new paradigm for biology
- Report on horizontal gene transfer by Mae-Wan Ho, 1999
- Recent evidence confirms risks of horizontal gene transfer