Electric dipole: Difference between revisions
imported>Paul Wormer |
mNo edit summary |
||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
In [[chemistry]], an '''electric dipole''' is a charge distribution consisting of two electric point charges of opposite sign and equal magnitude. | In [[chemistry]], an '''electric dipole''' is a charge distribution consisting of two electric point charges of opposite sign and equal magnitude. | ||
Usually it is added to this definition that the distance ''r'' between the two point charges is small—if this is the case the dipole is a '''point dipole'''. ''Small'' is a relative concept and requires further specification. In general one is interested in the electric potential field due to the dipolar charge distribution at a distance ''R'' from the midpoint of the dipole. If it holds that | |||
''r'' << ''R'' then, seen from the distance ''R'', the dipolar charge distribution | ''r'' << ''R'' then, seen from the distance ''R'', the dipolar charge distribution can be considered to be a ''point dipole''. | ||
In [[physics]], one meets the following related, but different, definition: an '''electric dipole''' of an electrostatic charge distribution ρ('''r''') is its first [[moment]], | In [[physics]], one meets the following related, but different, definition: an '''electric dipole moment''' (often briefly "dipole") of an electrostatic charge distribution ρ('''r''') is its first [[moment]], | ||
:<math> | :<math> | ||
\boldsymbol{\mu} \;\stackrel{\mathrm{def}}{=}\; \int\, \mathbf{r}\, \rho(\mathbf{r})\, \mathrm{d}\mathbf{r} | \boldsymbol{\mu} \;\stackrel{\mathrm{def}}{=}\; \int\, \mathbf{r}\, \rho(\mathbf{r})\, \mathrm{d}\mathbf{r} | ||
| Line 24: | Line 24: | ||
where we used ''q''<sub>1</sub> = −|''q''| and ''q''<sub>2</sub> = |''q''|. | where we used ''q''<sub>1</sub> = −|''q''| and ''q''<sub>2</sub> = |''q''|. | ||
This leads to | This leads to another possible definition: '''an electric dipole moment''' is a vector pointing from a negative electric point charge to a positive electric point charge, where both charges are of the same magnitude. The size (length) of the dipole is the distance between the point charges times the magnitude of the charges. Often, especially in chemistry, this size (a scalar) is also referred to as "dipole moment". | ||
In chemistry sizes of molecular dipole moments are often quoted to indicate the amount of charge separation in a [[molecule]]. Usually chemists are only interested in absolute values, a large dipole indicates a highly polar molecule (much separation of charge), whereas non-polar molecules have negligible dipoles. In the rare | In chemistry sizes of molecular dipole moments are often quoted to indicate the amount of charge separation in a [[molecule]]. Usually chemists are only interested in absolute values, a large dipole indicates a highly polar molecule (much separation of charge), whereas non-polar molecules have negligible dipoles. In the rare cases that the sign of a dipole plays a role in chemistry, most (organic) chemists use a convention that is opposite to the convention just discussed: they let the dipole point from a positively charged region in the molecule to a negatively charged region. | ||
==Field of point dipole== | ==Field of point dipole== | ||
The potential field of a point dipole '''μ''' | {{Image|Dipole field.png|right|300px|Fig. 1 Field of two point charges}} | ||
The potential field at a point '''R''' of a point dipole '''μ''' is given by (see figure 1 for the definition of <font style="vertical-align: top"><math> \vec{\mathbf{R}}</math></font> ≡ '''R'''): | |||
:<math> | :<math> | ||
V_\mathrm{dip}(\mathbf{R}) = \frac{\boldsymbol{\mu}\cdot\mathbf{R}}{4\pi \epsilon_0\,R^3} | V_\mathrm{dip}(\mathbf{R}) = \frac{\boldsymbol{\mu}\cdot\mathbf{R}}{4\pi \epsilon_0\,R^3} | ||
\qquad\qquad\qquad\qquad\qquad\qquad(1) | |||
</math> | </math> | ||
where ''R'' is the length of '''R''', the dot indicates the [[dot product]] between '''μ''' and '''R''', and ε<sub>0</sub> is the [[electric constant]] (vacuum permittivity). | where ''R'' is the length of '''R''', the dot indicates the [[dot product]] between '''μ''' and '''R''', and ε<sub>0</sub> is the [[electric constant]] (vacuum permittivity). | ||
The potential ''V''<sub>dip</sub>('''R''') is a special term in a general expansion; see for the general form the article on the [[multipole expansion of electric field|multipole expansion]] of an electric field | The potential ''V''<sub>dip</sub>('''R''') is a special term in a general expansion; see for the general form the article on the [[multipole expansion of electric field|multipole expansion]] of an electric field. | ||
===Proof=== | |||
The potential field of a point dipole can be determined fairly easily, see | The equipotential surfaces for the case '''μ''' = (0, 0, 1) in [[Gaussian units]] (4πε<sub>0</sub>=1) are shown in figure 2. The Matlab<sup>®</sup> code that generates figure 2 is on the [[Electric dipole/Code|code subpage]]. | ||
===Proof of equation (1)=== | |||
The potential field of a point dipole can be determined fairly easily, see figure 1 for the symbols. In the equations we indicate vectors with bold letters, omitting the arrows on top. The length of a vector is indicated with the corresponding symbol in italics. Write | |||
:<math> | :<math> | ||
\begin{align} | \begin{align} | ||
| Line 48: | Line 52: | ||
\end{align} | \end{align} | ||
</math> | </math> | ||
{{Image|Dipole field.png|right|300px| | {{Image|Dipole potential field.png|right|300px|Fig. 2. Equipotential surfaces of a point dipole in the origin. Positive and negative charge on positive and negative ''z''-axis, respectively. Plotted is <math>\scriptstyle z/(\sqrt{z^2+x^2})^3</math> }} | ||
Use the expansions, valid for ''x'' << 1, | Use the expansions, valid for ''x'' << 1, | ||
:<math> | :<math> | ||
| Line 73: | Line 78: | ||
\quad \hbox{and}\quad \boldsymbol{\mu} \equiv q \mathbf{r}. | \quad \hbox{and}\quad \boldsymbol{\mu} \equiv q \mathbf{r}. | ||
</math> | </math> | ||
==Dipole in an electric field== | |||
Consider a continuous charge distribution ρ('''r'''). If this charge distribution is non-polarizable its interaction energy with an external electrostatic potential ''V''('''r''') is | |||
:<math> | |||
E = \int \rho(\mathbf{r}) V(\mathbf{r}) d\mathbf{r},\, | |||
</math> | |||
where the integral extends over ρ. Very often the charge distribution is very small and ''V''('''r''') may be assumed to change linearly over the extents of ρ('''r'''). For instance, | |||
if the electric field is of macroscopic origin and the charge distribution is microscopic, i.e., an atom or a molecule, it is reasonable to linearize ''V'' over the charge distribution. ''V'' is then given by a two-term [[Taylor series|Taylor expansion]], | |||
:<math> | |||
V(\mathbf{r}) \approx V(\mathbf{0}) - \sum_{\alpha=x,y,z} r_\alpha F_\alpha \quad \hbox{with}\quad | |||
F_\alpha \equiv - \left(\frac{\partial V}{\partial r_\alpha} \right)_{\mathbf{0}}, | |||
</math> | |||
where we took the origin '''0''' somewhere within ρ. The electric field '''F''' is a homogeneous [[vector field]]. With ''V''('''0''') as the zero of potential, the interaction becomes | |||
:<math> | |||
E \approx - \sum_{\alpha=x,y,z} F_\alpha \int \rho(\mathbf{r}) r_\alpha d\mathbf{r} \equiv | |||
- \sum_{\alpha=x,y,z} F_\alpha \mu_\alpha = - \mathbf{F}\cdot \boldsymbol{\mu}. | |||
</math> | |||
Here we meet again the first moment '''μ''' of the charge distribution ρ('''r'''), the dipole moment of ρ('''r'''). If linearizing ''V'' is too drastic an approximation, more terms in the Taylor expansion may be included, this leads to a [[multipole expansion of electric field|multipole expansion of the potential]] ''V''('''r''').[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 07:00, 11 August 2024
In chemistry, an electric dipole is a charge distribution consisting of two electric point charges of opposite sign and equal magnitude.
Usually it is added to this definition that the distance r between the two point charges is small—if this is the case the dipole is a point dipole. Small is a relative concept and requires further specification. In general one is interested in the electric potential field due to the dipolar charge distribution at a distance R from the midpoint of the dipole. If it holds that r << R then, seen from the distance R, the dipolar charge distribution can be considered to be a point dipole.
In physics, one meets the following related, but different, definition: an electric dipole moment (often briefly "dipole") of an electrostatic charge distribution ρ(r) is its first moment,
In this definition a dipole is a vector depending on the characteristics of the charge distribution.
Combining the two definitions, one considers two point charges: q1 at position r1 and q2 at position r2. The charge distribution is written in terms of Dirac delta functions
where the charges are of opposite sign and equal magnitude. The first moment of this charge distribution is
where we used q1 = −|q| and q2 = |q|.
This leads to another possible definition: an electric dipole moment is a vector pointing from a negative electric point charge to a positive electric point charge, where both charges are of the same magnitude. The size (length) of the dipole is the distance between the point charges times the magnitude of the charges. Often, especially in chemistry, this size (a scalar) is also referred to as "dipole moment".
In chemistry sizes of molecular dipole moments are often quoted to indicate the amount of charge separation in a molecule. Usually chemists are only interested in absolute values, a large dipole indicates a highly polar molecule (much separation of charge), whereas non-polar molecules have negligible dipoles. In the rare cases that the sign of a dipole plays a role in chemistry, most (organic) chemists use a convention that is opposite to the convention just discussed: they let the dipole point from a positively charged region in the molecule to a negatively charged region.
Field of point dipole
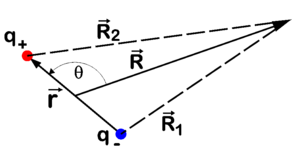
The potential field at a point R of a point dipole μ is given by (see figure 1 for the definition of ≡ R):
where R is the length of R, the dot indicates the dot product between μ and R, and ε0 is the electric constant (vacuum permittivity).
The potential Vdip(R) is a special term in a general expansion; see for the general form the article on the multipole expansion of an electric field.
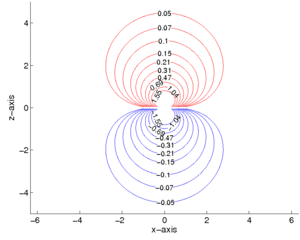
The equipotential surfaces for the case μ = (0, 0, 1) in Gaussian units (4πε0=1) are shown in figure 2. The Matlab® code that generates figure 2 is on the code subpage.
Proof of equation (1)
The potential field of a point dipole can be determined fairly easily, see figure 1 for the symbols. In the equations we indicate vectors with bold letters, omitting the arrows on top. The length of a vector is indicated with the corresponding symbol in italics. Write
Use the expansions, valid for x << 1,
Since r << R it follows that r/R << 1 and hence
The Coulomb potential at the point R is the sum of the potentials due to the two charges, which are of equal magnitude q and of opposite sign,
where we used
Dipole in an electric field
Consider a continuous charge distribution ρ(r). If this charge distribution is non-polarizable its interaction energy with an external electrostatic potential V(r) is
where the integral extends over ρ. Very often the charge distribution is very small and V(r) may be assumed to change linearly over the extents of ρ(r). For instance, if the electric field is of macroscopic origin and the charge distribution is microscopic, i.e., an atom or a molecule, it is reasonable to linearize V over the charge distribution. V is then given by a two-term Taylor expansion,
where we took the origin 0 somewhere within ρ. The electric field F is a homogeneous vector field. With V(0) as the zero of potential, the interaction becomes
Here we meet again the first moment μ of the charge distribution ρ(r), the dipole moment of ρ(r). If linearizing V is too drastic an approximation, more terms in the Taylor expansion may be included, this leads to a multipole expansion of the potential V(r).


![{\displaystyle {\boldsymbol {\mu }}\equiv \int \,\mathbf {r} \,\rho (\mathbf {r} )\,\mathrm {d} \mathbf {r} =\int \,\mathbf {r} \,{\big [}q_{1}\delta (\mathbf {r} -\mathbf {r} _{1})+q_{2}\delta (\mathbf {r} -\mathbf {r} _{2}){\big ]}\,\mathrm {d} \mathbf {r} =q_{1}\mathbf {r} _{1}+q_{2}\mathbf {r} _{2}=|q|\,(\mathbf {r} _{2}-\mathbf {r} _{1}),}](https://wikimedia.org/api/rest_v1/media/math/render/svg/70649c77add49eb7fc6e089905417183288db7a1)


![{\displaystyle {\begin{aligned}\mathbf {R} _{1}&=\mathbf {R} +{\frac {1}{2}}\mathbf {r} &\Longrightarrow &|\mathbf {R} _{1}|^{2}={\Big (}\mathbf {R} +{\frac {1}{2}}\mathbf {r} {\Big )}\cdot {\Big (}\mathbf {R} +{\frac {1}{2}}\mathbf {r} {\Big )}=R^{2}\left[1+{\Big (}{\frac {r}{2R}}{\Big )}^{2}+{\frac {r}{R}}\cos \theta \right]\\\mathbf {R} _{2}&=\mathbf {R} -{\frac {1}{2}}\mathbf {r} &\Longrightarrow &|\mathbf {R} _{1}|^{2}={\Big (}\mathbf {R} -{\frac {1}{2}}\mathbf {r} {\Big )}\cdot {\Big (}\mathbf {R} -{\frac {1}{2}}\mathbf {r} {\Big )}=R^{2}\left[1+{\Big (}{\frac {r}{2R}}{\Big )}^{2}-{\frac {r}{R}}\cos \theta \right]\\\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1d76fc169cd5d0feeca7cbf9635092312ff364bb)



![{\displaystyle {\begin{aligned}V(\mathbf {R} )\equiv &{\frac {q_{-}}{4\pi \epsilon _{0}\,|\mathbf {R} _{1}|}}+{\frac {q_{+}}{4\pi \epsilon _{0}\,|\mathbf {R} _{2}|}}\\\approx &{\frac {q}{4\pi \epsilon _{0}\,R}}\left[-1+{\frac {r}{2R}}\cos \theta +1+{\frac {r}{2R}}\cos \theta \right]={\frac {q\;\mathbf {r} \cdot \mathbf {R} }{4\pi \epsilon _{0}\,R^{3}}}={\frac {{\boldsymbol {\mu }}\cdot \mathbf {R} }{4\pi \epsilon _{0}\,R^{3}}}\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/86ff40888871b1dd5679838765241f51abd1bb21)



