Microsporum canis: Difference between revisions
imported>Michelle Jaramillo Osorio |
mNo edit summary |
||
| (32 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{Taxobox | {{Taxobox | ||
| color = pink | | color = pink | ||
| name = | | name = ''Microsporum canis'' | ||
| image = | | image = Macroconidia_Microsporum_canis.JPG | ||
| regnum = Fungi | | regnum = Fungi | ||
| phylum = Ascomycota | | phylum = Ascomycota | ||
| Line 17: | Line 15: | ||
}} | }} | ||
''Microsporum canis'' is a [[fungus]] also known as a dermatophyte that causes [[dermatophytosis]] ([[ringworm]]) in | '''''Microsporum canis''''' is a [[fungus]] also known as a dermatophyte that causes [[dermatophytosis]] ([[ringworm]]) in dogs and cats. They are commonly found in humid, warm climates. Although canines and felines are its natural reservoir it can cause ringworm in humans. | ||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
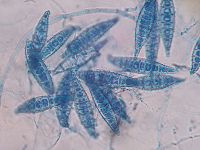
Microscopically this species has multicelled [[macroconidia]] (spores) with thick, rough walls. They are barreled shaped with an asymmetrical apical knob and are 6 to 15 cells long. When grown on a culture medium for at least 4 days colonies produce a white cottony surface with yellow around the periphery and the underside is bright yellow or orange. | Microscopically this species has multicelled [[macroconidia]] (spores) with thick, rough walls. They are barreled shaped with an asymmetrical apical knob and are 6 to 15 cells long. When grown on a [[culture medium]] for at least 4 days colonies produce a white cottony surface with yellow around the periphery and the underside is bright yellow or orange. <sup>2</sup> | ||
This organism gains energy from [[keratin]] found in nails, hair and skin. It secretes [[keratinolytic protease]], which provides the fungus with nutrients by degrading keratin into easily assimilable [[metabolites]]. This secreted protease also allows the invasion of keratinized structures and controls the host defense mechanisms. | This organism gains energy from [[keratin]] found in nails, hair and skin. It secretes [[keratinolytic protease]], which provides the fungus with nutrients by degrading keratin into easily assimilable [[metabolites]]. This secreted protease also allows the invasion of keratinized structures and controls the host defense mechanisms. <sup>4</sup> | ||
==Pathology== | ==Pathology== | ||
This fungal [[pathogen]] causes ringworm mainly in cats and dogs, but is a common source for human infection. This fungus can be picked up by direct contact with other infected animals or by contact with spores that have contaminated objects such as grooming equipment, furniture and the environment. Spores are very resistant without treatment and can live up to two years infecting animals and humans. Spores will attach to the skin and germinate producing | This fungal [[pathogen]] causes ringworm mainly in cats and dogs, but is a common source for human infection. This fungus can be picked up by direct contact with other infected animals or by contact with [[arthrospores]]([[asexual]] spores formed in the [[hyphae]] of the [[parasitic]] stage) <sup>5</sup> that have contaminated objects such as grooming equipment, furniture and the environment. Spores are very resistant without treatment and can live up to two years infecting animals and humans. Spores will attach to the skin and germinate producing hyphae, which will then grow in the dead, superficial layers of the skin, hair or nails. | ||
The most investigated [[virulence]] factors has been keratinolytic protease. The secretion of this protease causes some damage to the skin and hair follicle. The skin will have a hypersensitive reaction becoming inflamed causing the fungus to move away from the site to normal skin. This creates characteristic circular lesions with healing at the center and inflammation at the edge. | The most investigated [[virulence]] factors has been keratinolytic protease. The secretion of this protease causes some damage to the skin and hair [[follicle]]. The skin will have a hypersensitive reaction becoming inflamed causing the fungus to move away from the site to normal skin. This creates characteristic circular lesions with healing at the center and inflammation at the edge.<sup>3</sup> | ||
Some cats can be [[asymptomatic]] while others will exhibit flacky skin, hair lose and occasionally inflamed skin. Cats that are less than a year old, long haired or have a comprised immune system are more susceptible to ringworm. | Some cats can be [[asymptomatic]] while others will exhibit flacky skin, hair lose and occasionally inflamed skin. Cats that are less than a year old, long haired or have a comprised immune system are more susceptible to ringworm. | ||
A reason for younger cats susceptibility to this fungus can be that their immune system is immature and its defense mechanisms are limited to infection. Long haired cats are easier targets for infection because their grooming is less efficient and self grooming is an effective way of removing spores from the skin and fur. Their long hair also protects the dermatophytes from sun exposure which they can not tolerate. The infection is primarily found on ears head or extremity of the paws. | A reason for younger cats susceptibility to this fungus can be that their immune system is immature and its defense mechanisms are limited to infection. Long haired cats are easier targets for infection because their grooming is less efficient and self grooming is an effective way of removing spores from the skin and fur. Their long hair also protects the dermatophytes from sun exposure which they can not tolerate. The infection is primarily found on ears head or extremity of the paws. <sup>1</sup> | ||
Dogs will exhibit non-inflammatory flaky skin with hair lose. | Dogs will exhibit non-inflammatory flaky skin with hair lose. | ||
== | ==Current Research== | ||
1) ''Microsporum canis'' is a parasitic fungus that secretes keratinolytic protease to invade keratinized structures of hair, skin and nails in humans and animals. They cause dermatophytosis in mainly dogs and cats, but human infections are increasing, especially in many European countries. A reason for increased infections in humans is due to asymptomatic infected cats. | |||
Most research has been conducted on keratinolytic protease, which is secreted by this fungus. There is evidence of it being a potential virulence factor by controlling the host’s defense mechanisms. Keratinolytic protease also provides the fungus nutrients by degrading keratin structures into easily absorbable [[metabolites]]. | |||
M. canis secretes a keratinolytic [[subtilisin]]-like protease called 31.5 kDa. It also secretes three subtilisin-like proteases (SUBs), SUB1, SUB2 and SUB3. An in [[vitro expression]] of this protease has been studied much more than the in [[vivo expression]]. The isolation and characterization of these three SUBs allow for the study of their potential role of pathogenesis of M. canis dermatophytosis in the in vivo transcription of their genes, which is demonstrated in the hair of experimentally infected guinea pigs. | |||
The detection of the in vivo [[transcription]] of SUB1, SUB2, and SUB3 was seen on [[agarose gel]] as bands. This was not detected under the same conditions in control extracts. Obtaining [[RT-nested]] [[PCR]] products, from total [[RNA]] extracted of M. canis infected hair of guinea pigs allowed for this procedure. The results revealed [[homology]] between SUB1, SUB2, and SUB3 by the identification of the [[catalytic triad]], conserved residues typical of [[serine]] proteases of the subtilisin family and by the high [[amino acid]] identity percentage. This suggests that M. canis contains a family of SUBs. Proteases encoded by a gene family have been shown to be related to virulence in other fungal infections and this would be evidence of M. canis pathogenic infection. The article states that further research needs to be conducted on the fungal infection of M. canis. <sup>4</sup> | |||
2) This study is based on examining the correlation between the presence of infective arthrospores on the hair coat of dogs and cats without [[cutaneous]] lesions and the frequency of owners becoming infected. Many cases of tinea corporis in southern Italy are due to the exposure of infected asymptomatic animals, especially cats. Age, sex, breed, habitat and season were also evaluated in this study as potential risk factors of infection. | |||
Other research has shown cats to be the major source of infection in humans and other animals. Infected cats shed a greater amount of spores in the environment than dogs and this contributes greatly to human infection. This study has shown that asymptomatic dogs can also be carriers of M. canis and [[geophilic]] species on their coats. Humans that were diagnosed with tinea corporis were cohabiting with dogs and cats that carried M. canis on their hair coats. In some cases healthy owners were living with infected cats but this was never seen with dogs. This would indicate that cats were the source of transmission of M. canis to humans. Another part of this study indicated that some infected dog owners were becoming infected from a common source and infecting their dogs. | |||
The risk factors that proved to have an effect on cats becoming carries of M. canis were age, habitat and season. Cats younger than a year and hair samples taken from cats during the winter months contributed to a higher frequency of infection. The majority of examined cats lived in ([[rural]]) areas where they had access to the outdoors, which increased their chances of infection by possible contact with wild animals. On the other hand dogs were found to have a higher presence of geophilic dermatophytes than M. canis. This can be explained by the warm climate and that almost half of the examined dogs lived outdoors. The outcome of this study revealed the difference between the role of dogs and cats as a carrier for M. canis.<sup>6</sup> | |||
3) The purpose of this study was to use a simple [[extraction]]/PCR protocol to help identify fungal species found in dermatophytic [[pseudomycetoma]] (DPM). DPM is a deep [[dermal]]/[[subcuntaneous]] infection of humans and animals with [[pyogranulomatous]] reaction. The rupture of the follicle and a build up of dermis or [[subcutis]] tissue causes these lesions. | |||
M. canis | ''Microsporum canis'' is the main fungus that causes DPM. It has been seen in specific dog and cat breeds such as [[Yorkshire terriers]] and [[Persian]] cats. Identifying microscopic features of M. canis is a way of diagnosing DPM. There are other tests that can be used to identify the fungus causing infection, such as [[fungal cultures]], dermatological [[specimens]] (scales and skin swabs) and [[paraffin]]-embedded human skin samples. Simple extraction/PCR protocol is a test that has never been used in cases of DPM. This test is followed by [[amplification]] of PCR products in paraffin-embedded tissues obtained from cases of DPM. [[Sequencing]] the products allows for the identification of the fungal species. | ||
All healthy samples of paraffin-embedded tissue gave negative results from the PCR amplification, while all the samples diagnosed as DPM were positive. M. canis showed 100% identity in products that were sequenced from positive samples. | |||
This has been the first study to identify the presence of dermatophytic DNA from DPM cases. The PCR test proves to be helpful in providing useful diagnostic information in deep infections. This test is the best alternative to fungal cultures when studying the agents of DPM from specimens.<sup>7</sup> | |||
==References== | ==References== | ||
<references/> | <references/> | ||
1) http://www.fabcats.org/owners/skin/ringworm.html | 1) http://www.fabcats.org/owners/skin/ringworm.html | ||
2) http://www.doctorfungus.org/thefungi/microsporum_canis.htm | |||
4)http://www. | |||
3)http://www.vetstreamfelis.com/ACI/January08/VMD1/bug00270.asp | |||
4)Descamps et al. Isolation of a Microsporum canis Gene Family Encoding Three Subtilisin-Like Proteases Expressed in vivo. Journal of Investigative Dermatology 2002; 119: 830–835 | |||
5) Institute for International Cooperation in Animal Biologics | |||
http://www.cfsph.iastate.edu/Factsheets/pdfs/dermatophytosis.pdf | |||
6) Cafarchia et al. Isolation of ''Microsporum canis'' from the hair coat of pet dogs and cats belonging to owners diagnosed with M. canis tinea corporis. European Society of Veterinary Dermatology 2006; 17: 327-331 | |||
7) Nardoni et al. Identification of Microsporum canis from dermatophytic pseudomycetoma in paraffin-embedded veterinary specimens using a common PCR protocol. Blackwell Publishing Ltd 2007; 50: 215-217.[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 07:01, 19 September 2024
| Microsporum canis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Microsporum canis |
Microsporum canis is a fungus also known as a dermatophyte that causes dermatophytosis (ringworm) in dogs and cats. They are commonly found in humid, warm climates. Although canines and felines are its natural reservoir it can cause ringworm in humans.
Cell structure and metabolism
Microscopically this species has multicelled macroconidia (spores) with thick, rough walls. They are barreled shaped with an asymmetrical apical knob and are 6 to 15 cells long. When grown on a culture medium for at least 4 days colonies produce a white cottony surface with yellow around the periphery and the underside is bright yellow or orange. 2 This organism gains energy from keratin found in nails, hair and skin. It secretes keratinolytic protease, which provides the fungus with nutrients by degrading keratin into easily assimilable metabolites. This secreted protease also allows the invasion of keratinized structures and controls the host defense mechanisms. 4
Pathology
This fungal pathogen causes ringworm mainly in cats and dogs, but is a common source for human infection. This fungus can be picked up by direct contact with other infected animals or by contact with arthrospores(asexual spores formed in the hyphae of the parasitic stage) 5 that have contaminated objects such as grooming equipment, furniture and the environment. Spores are very resistant without treatment and can live up to two years infecting animals and humans. Spores will attach to the skin and germinate producing hyphae, which will then grow in the dead, superficial layers of the skin, hair or nails. The most investigated virulence factors has been keratinolytic protease. The secretion of this protease causes some damage to the skin and hair follicle. The skin will have a hypersensitive reaction becoming inflamed causing the fungus to move away from the site to normal skin. This creates characteristic circular lesions with healing at the center and inflammation at the edge.3 Some cats can be asymptomatic while others will exhibit flacky skin, hair lose and occasionally inflamed skin. Cats that are less than a year old, long haired or have a comprised immune system are more susceptible to ringworm. A reason for younger cats susceptibility to this fungus can be that their immune system is immature and its defense mechanisms are limited to infection. Long haired cats are easier targets for infection because their grooming is less efficient and self grooming is an effective way of removing spores from the skin and fur. Their long hair also protects the dermatophytes from sun exposure which they can not tolerate. The infection is primarily found on ears head or extremity of the paws. 1 Dogs will exhibit non-inflammatory flaky skin with hair lose.
Current Research
1) Microsporum canis is a parasitic fungus that secretes keratinolytic protease to invade keratinized structures of hair, skin and nails in humans and animals. They cause dermatophytosis in mainly dogs and cats, but human infections are increasing, especially in many European countries. A reason for increased infections in humans is due to asymptomatic infected cats.
Most research has been conducted on keratinolytic protease, which is secreted by this fungus. There is evidence of it being a potential virulence factor by controlling the host’s defense mechanisms. Keratinolytic protease also provides the fungus nutrients by degrading keratin structures into easily absorbable metabolites.
M. canis secretes a keratinolytic subtilisin-like protease called 31.5 kDa. It also secretes three subtilisin-like proteases (SUBs), SUB1, SUB2 and SUB3. An in vitro expression of this protease has been studied much more than the in vivo expression. The isolation and characterization of these three SUBs allow for the study of their potential role of pathogenesis of M. canis dermatophytosis in the in vivo transcription of their genes, which is demonstrated in the hair of experimentally infected guinea pigs.
The detection of the in vivo transcription of SUB1, SUB2, and SUB3 was seen on agarose gel as bands. This was not detected under the same conditions in control extracts. Obtaining RT-nested PCR products, from total RNA extracted of M. canis infected hair of guinea pigs allowed for this procedure. The results revealed homology between SUB1, SUB2, and SUB3 by the identification of the catalytic triad, conserved residues typical of serine proteases of the subtilisin family and by the high amino acid identity percentage. This suggests that M. canis contains a family of SUBs. Proteases encoded by a gene family have been shown to be related to virulence in other fungal infections and this would be evidence of M. canis pathogenic infection. The article states that further research needs to be conducted on the fungal infection of M. canis. 4
2) This study is based on examining the correlation between the presence of infective arthrospores on the hair coat of dogs and cats without cutaneous lesions and the frequency of owners becoming infected. Many cases of tinea corporis in southern Italy are due to the exposure of infected asymptomatic animals, especially cats. Age, sex, breed, habitat and season were also evaluated in this study as potential risk factors of infection.
Other research has shown cats to be the major source of infection in humans and other animals. Infected cats shed a greater amount of spores in the environment than dogs and this contributes greatly to human infection. This study has shown that asymptomatic dogs can also be carriers of M. canis and geophilic species on their coats. Humans that were diagnosed with tinea corporis were cohabiting with dogs and cats that carried M. canis on their hair coats. In some cases healthy owners were living with infected cats but this was never seen with dogs. This would indicate that cats were the source of transmission of M. canis to humans. Another part of this study indicated that some infected dog owners were becoming infected from a common source and infecting their dogs.
The risk factors that proved to have an effect on cats becoming carries of M. canis were age, habitat and season. Cats younger than a year and hair samples taken from cats during the winter months contributed to a higher frequency of infection. The majority of examined cats lived in (rural) areas where they had access to the outdoors, which increased their chances of infection by possible contact with wild animals. On the other hand dogs were found to have a higher presence of geophilic dermatophytes than M. canis. This can be explained by the warm climate and that almost half of the examined dogs lived outdoors. The outcome of this study revealed the difference between the role of dogs and cats as a carrier for M. canis.6
3) The purpose of this study was to use a simple extraction/PCR protocol to help identify fungal species found in dermatophytic pseudomycetoma (DPM). DPM is a deep dermal/subcuntaneous infection of humans and animals with pyogranulomatous reaction. The rupture of the follicle and a build up of dermis or subcutis tissue causes these lesions.
Microsporum canis is the main fungus that causes DPM. It has been seen in specific dog and cat breeds such as Yorkshire terriers and Persian cats. Identifying microscopic features of M. canis is a way of diagnosing DPM. There are other tests that can be used to identify the fungus causing infection, such as fungal cultures, dermatological specimens (scales and skin swabs) and paraffin-embedded human skin samples. Simple extraction/PCR protocol is a test that has never been used in cases of DPM. This test is followed by amplification of PCR products in paraffin-embedded tissues obtained from cases of DPM. Sequencing the products allows for the identification of the fungal species.
All healthy samples of paraffin-embedded tissue gave negative results from the PCR amplification, while all the samples diagnosed as DPM were positive. M. canis showed 100% identity in products that were sequenced from positive samples.
This has been the first study to identify the presence of dermatophytic DNA from DPM cases. The PCR test proves to be helpful in providing useful diagnostic information in deep infections. This test is the best alternative to fungal cultures when studying the agents of DPM from specimens.7
References
1) http://www.fabcats.org/owners/skin/ringworm.html
2) http://www.doctorfungus.org/thefungi/microsporum_canis.htm
3)http://www.vetstreamfelis.com/ACI/January08/VMD1/bug00270.asp
4)Descamps et al. Isolation of a Microsporum canis Gene Family Encoding Three Subtilisin-Like Proteases Expressed in vivo. Journal of Investigative Dermatology 2002; 119: 830–835
5) Institute for International Cooperation in Animal Biologics http://www.cfsph.iastate.edu/Factsheets/pdfs/dermatophytosis.pdf
6) Cafarchia et al. Isolation of Microsporum canis from the hair coat of pet dogs and cats belonging to owners diagnosed with M. canis tinea corporis. European Society of Veterinary Dermatology 2006; 17: 327-331
7) Nardoni et al. Identification of Microsporum canis from dermatophytic pseudomycetoma in paraffin-embedded veterinary specimens using a common PCR protocol. Blackwell Publishing Ltd 2007; 50: 215-217.