Periodic table of elements: Difference between revisions
imported>Peter Jackson |
imported>Paul Wormer |
||
| Line 12: | Line 12: | ||
== Elements by periodic table group (vertical column) == | == Elements by periodic table group (vertical column) == | ||
Elements in any one Group behave in a similar way and show the same overall general properties. The number of electrons in the outer shells of the electron orbitals is the same within a group, only the quantum number describing the orbitals increases. | |||
:''See [[Atomic electron configuration]] for the orbital occupancies of ground state atoms.'' | |||
Elements in any one Group behave in a similar way and show the same overall general properties. The number of electrons in the outer shells of the electron orbitals is the same within a group, only the quantum number describing the orbitals increases. For instance, the right column is occupied by the noble gases. Because they have an outer shell of electrons that is completely filled, they are inert in behaviour. (See the article [[Atomic orbital#Solutions of the atomic Schrödinger equation|atomic orbital]] for a more detailed explanation of the building up of the electronic shells.) On the extreme left there is a group of metals, called the alkali metals. They are characterized by having a single electron in their outer valence orbital. Going down from [[Lithium|Li]], [[Sodium|Na]], [[Potassium|K]] to [[Francium|Fr]] the reactivity decreases, but these metals react easily – for example, with water, in a very exothermic way. This lowered reactivity is related to the atomic weight. As the [[mass]] of the nucleus increases with each neutron and proton, so also does the number of electrons (to balance the electric charge). Because these electrons are all at a greater distance from the nucleus, the energy gained from removing one electron diminishes according to the proportionality of ''r''<sup>−2</sup>. The one free electron in the outer valence shell is in a so-called ''s''-[[Hydrogen-like atom#Quantum numbers of hydrogen-like wave functions|orbital]]. In all these metals there is only one free electron and these ball-shaped orbitals are denoted by 1''s'', 2''s'', 3''s'', etc., all with an increasing average radius from the nucleus. For all the subsequent groups, characterizations can be formulated based upon similarities in reactivity — the way they react, how many electrons can be shared, etc. As a result, the likelihood of reactants to be able to react together can be determined from the Periodic Table of Elements. | |||
The way the element at the top of a column reacts and the way it produces new chemicals give a clue to how other elements in that same group (column) will react. For instance [[carbon]] and [[hydrogen]] (H<sub>2</sub>) react to form [[methane]]. The bonds of the [[Carbon|carbon (C)]] take a tetrahedral or pyramidal shape. [[Silicon]] or [[Silicon|Si]] responds in the same way and carbon-chemistry is to a significant extent the same as silicon-chemistry. | The way the element at the top of a column reacts and the way it produces new chemicals give a clue to how other elements in that same group (column) will react. For instance [[carbon]] and [[hydrogen]] (H<sub>2</sub>) react to form [[methane]]. The bonds of the [[Carbon|carbon (C)]] take a tetrahedral or pyramidal shape. [[Silicon]] or [[Silicon|Si]] responds in the same way and carbon-chemistry is to a significant extent the same as silicon-chemistry. | ||
Revision as of 23:08, 5 June 2009
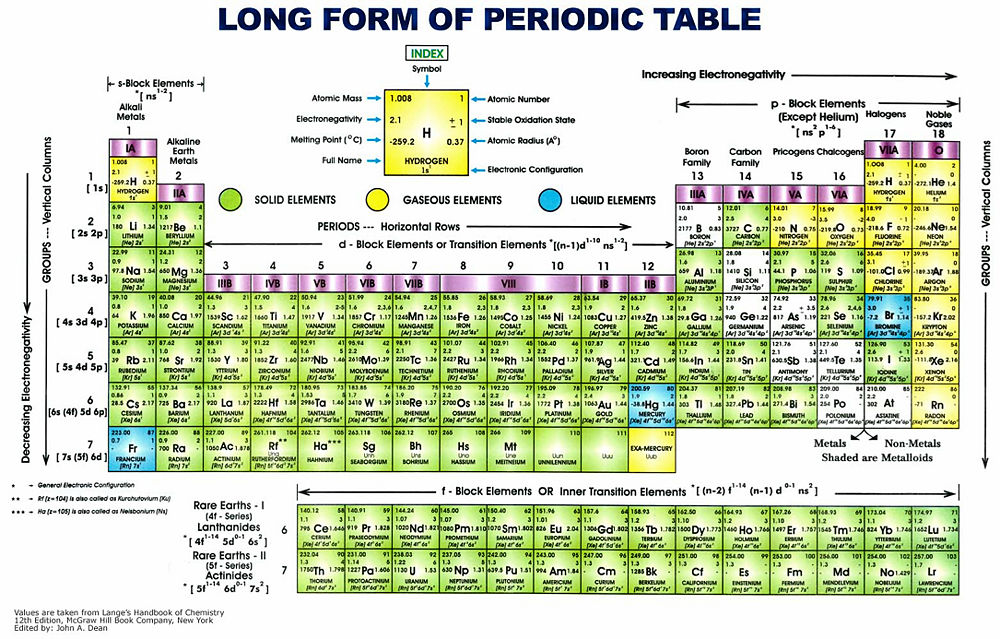
The Periodic Table of the Elements is a tabular method of displaying the elements. An element is a fundamental classification of atomic matter where differentiation of particles is based on the number of protons found in their nuclei. So far, 118 elements are known to exist either by producing them artificially or finding them naturally in the environment.
The component parts of the table are known as Rows and Groups (the later referring to columns). In the table, elements are sorted in ascending order of the number of protons in the nucleus (atomic number). The physical state of matter that they exist in at 273.15 K and 100,000 Pa is usually also provided.
Typical version
The table shown below is only one of many versions. Another one, different from the one below, is available online.[1]
Elements by periodic table group (vertical column)
- See Atomic electron configuration for the orbital occupancies of ground state atoms.
Elements in any one Group behave in a similar way and show the same overall general properties. The number of electrons in the outer shells of the electron orbitals is the same within a group, only the quantum number describing the orbitals increases. For instance, the right column is occupied by the noble gases. Because they have an outer shell of electrons that is completely filled, they are inert in behaviour. (See the article atomic orbital for a more detailed explanation of the building up of the electronic shells.) On the extreme left there is a group of metals, called the alkali metals. They are characterized by having a single electron in their outer valence orbital. Going down from Li, Na, K to Fr the reactivity decreases, but these metals react easily – for example, with water, in a very exothermic way. This lowered reactivity is related to the atomic weight. As the mass of the nucleus increases with each neutron and proton, so also does the number of electrons (to balance the electric charge). Because these electrons are all at a greater distance from the nucleus, the energy gained from removing one electron diminishes according to the proportionality of r−2. The one free electron in the outer valence shell is in a so-called s-orbital. In all these metals there is only one free electron and these ball-shaped orbitals are denoted by 1s, 2s, 3s, etc., all with an increasing average radius from the nucleus. For all the subsequent groups, characterizations can be formulated based upon similarities in reactivity — the way they react, how many electrons can be shared, etc. As a result, the likelihood of reactants to be able to react together can be determined from the Periodic Table of Elements.
The way the element at the top of a column reacts and the way it produces new chemicals give a clue to how other elements in that same group (column) will react. For instance carbon and hydrogen (H2) react to form methane. The bonds of the carbon (C) take a tetrahedral or pyramidal shape. Silicon or Si responds in the same way and carbon-chemistry is to a significant extent the same as silicon-chemistry.
Elements by periodic table row
Elements in a row show different periodicities – such as electronegativities, increasing atomic mass, increasing number of protons and neutrons. The stability of these elements diminishes however with an increasing number of nucleons (protons and neutrons). These instabilities lead to radioactive decay as is evident from the actinides and lanthanides, the groups of the rare earths. They contain elements such as plutonium, radon and uranium, and, notorious since the poisoning scandal in the UK, polonium.
The ratio of protons to neutrons is important in determining the stability of elements. If the ratio is greater than 1, the probability increases that an element (or an isotope of an element) will be radioactive and unstable. The greater the ratio, the greater the probability that the element will be radioactive.
Other properties vary differently from the column-wise view of the periodic table of elements. Going downward, the reactivity decreases. Going along a row from left to right, the reactivity increases, and properties such as electronegativity increase as well. For the latter reason, the tendency to lose an electron to form a covalent bond decreases and turns into the tendency of needing one or more electrons to form a stable covalent bond, producing a more stable chemical substance or molecule.
The alkali metals are the most eager to lose an electron (they have the lowest electronegativity in the row) making them very reactive with water. The halogens are reactive for the opposite reason – they need to attract that electron to obtain a more stable electron-configuration. hydrofluoric acid is among the strongest acids known to mankind, where sodium hydroxide is one of the strongest bases — as an example. Electronegativity also is the source for the occurrence of electrical polarity in substances. In every molecule of water (H2O), the hydrogen is less inclined to keep its electron tightly bound. The oxygen, on the other hand, being more electronegative, likes to share electrons to create a more stable electron configuration. This results in the protons'(hydrogen) side of the molecule being slightly more positively charged ∆+, whereas the oxygen side is slightly more negatively charged 2∆−. Therefore water is a highly polar fluid with the capability of facilitating hydrogen bonding in solutions, which is a somewhat non-standard behaviour.
Elements classified alphabetically
- See Elements
History
(To be expanded by anyone with the requisite knowledge)
The periodic table was developed by Dmitri Mendeleev, though an earlier version by Newland anticipated some of it.