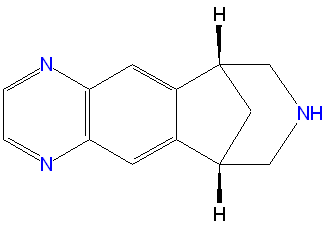
Varenicline
|
| |||||||
| varenicline | |||||||
| |||||||
| Uses: | smoking cessation | ||||||
| Properties: | nicotine receptor partial agonist | ||||||
| Hazards: | suicide, hostility, heart problems | ||||||
| |||||||
‘’’Varenicline’’’, sold under the brand names Chantix and Champix, is a medication which helps with smoking cessation by blocking nicotine receptors. Some concerns have been raised about possible side effects of the medication, prompting the FDA to add a Boxed Warning for Chantix’s label listing depressed mood, suicidal thoughts, and hostility, similar to warnings associated with Zyban (bupropion), a similar drug. More recently, a meta-analysis of 14 studies reported possible 72% increased risk of heart problems when taking varenicline.[1]
Mechanism of Action
Varenicline binds to neuronal nicotinic acetylcholine receptors, especially to the alpha-4/beta-2 receptor,[2],[3],[4] and stops nicotine from binding to these same receptors, thus removing satisfaction typically caused by smoke inhalation. In addition, varenicline is a weak agonist of these receptors, leading to a feeling of relaxation which lessens withdrawal symptoms of smoking cessation.
Drug toxicity
There is "the potential for an increased risk of serious adverse cardiovascular events associated with the use of varenicline among tobacco users" according to a meta-analysis. [5]
References
- ↑ Singh, S., Loke, Y.K., Spangler, J.G. and Furberg, C.D. (2011). "Risk of serious adverse cardiovascular events associated with varenicline: a systemic review and meta-analysis". Canadian Medical Association Journal. DOI:10.1503/cmaj.110218. Research Blogging.
- ↑ Coe et al (2005) Varenicline: an a4b2 nicotinic receptor partial agonist for smoking cessation. J.Med.Chem. 48 3474.
- ↑ Rollema et al (2007) Pharmacological profile of the a4b2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52 985
- ↑ Rollema et al (2009) Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem.Pharmacol. 78 813.
- ↑ Singh S, Loke YK, Spangler JG, Furberg CD (2011). "Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis.". CMAJ 183 (12): 1359-66. DOI:10.1503/cmaj.110218. PMID 21727225. PMC PMC3168618. Research Blogging. Review in: Ann Intern Med. 2011 Oct 18;155(8):JC4-5