Zalcitabine: Difference between revisions
imported>David E. Volk mNo edit summary |
imported>Meg Taylor m (spelling: supresses -> suppresses) |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{Chem infobox | |||
|align=right | |||
|image=[[Image:Zalcitabine strucure.jpg|center|thumb|200px]] | |||
|width=200px | |||
|molname=zalcitabine | |||
|synonyms= '''dideoxycytidine (ddC)''' | |||
|molformula= C<sub>9</sub>H<sub>13</sub>N<sub>3</sub>O<sub>3</sub> | |||
|molmass= 211.2178 | |||
|uses=HIV/AIDS | |||
|properties=RT inhibitor, cytosine analog, DNA terminator | |||
|hazards=see drug interactions | |||
|iupac= see chemistry section | |||
|casnumber=7481-89-2 | |||
}} | |||
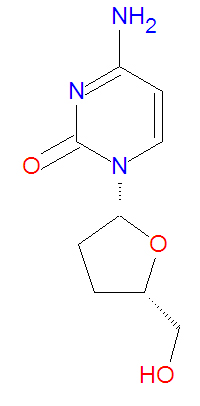
Its IUPAC chemical name is 4-amino-1-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one and it has molecular formula C<sub>9</sub>H<sub>13</sub>N<sub>3</sub>O<sub>3</sub>. | '''Zalcitabine''', or '''dideoxycytidine''' ('''DDC''' or '''DDCYD'''), is a dideoxynucleoside [[antiviral drug]] that is an analog of the natural DNA base [[cytosine]]. The lack of 3'-hydroxyl group makes it a viral DNA chain terminator. It also inhibits [[HIV]]-1 [[reverse transcriptase]] by binding to it and competing with the natural substrate [[deoxycytidine]] triphosphate (dCTP). The related drug [[lamivudine]], which has a sulfur atom in place of the 3'-carbon present in zalcitabine, suppresses viruses in a similar manner. | ||
== Chemistry == | |||
Its IUPAC chemical name of zalcitabine is 4-amino-1-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one, but it is also called dideoxycytidine (ddC), and it has molecular formula C<sub>9</sub>H<sub>13</sub>N<sub>3</sub>O<sub>3</sub> giving it a molecular mass of 211.2178 g/mol. It is a structural analog of the natural base deoxycytosine which used in DNA, but zalcitabine lacks the 3'-hydroxy group. | |||
== External links == | == External links == | ||
{{CZMed}} | |||
Latest revision as of 04:38, 14 February 2010
|
| |||||||
| zalcitabine | |||||||
| |||||||
| Uses: | HIV/AIDS | ||||||
| Properties: | RT inhibitor, cytosine analog, DNA terminator | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Zalcitabine, or dideoxycytidine (DDC or DDCYD), is a dideoxynucleoside antiviral drug that is an analog of the natural DNA base cytosine. The lack of 3'-hydroxyl group makes it a viral DNA chain terminator. It also inhibits HIV-1 reverse transcriptase by binding to it and competing with the natural substrate deoxycytidine triphosphate (dCTP). The related drug lamivudine, which has a sulfur atom in place of the 3'-carbon present in zalcitabine, suppresses viruses in a similar manner.
Chemistry
Its IUPAC chemical name of zalcitabine is 4-amino-1-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one, but it is also called dideoxycytidine (ddC), and it has molecular formula C9H13N3O3 giving it a molecular mass of 211.2178 g/mol. It is a structural analog of the natural base deoxycytosine which used in DNA, but zalcitabine lacks the 3'-hydroxy group.
External links
The most up-to-date information about Zalcitabine and other drugs can be found at the following sites.
- Zalcitabine - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Zalcitabine - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Zalcitabine - Detailed information from DrugBank.