User:David MacQuigg/Sandbox/Nuclear waste: Difference between revisions
| Line 1: | Line 1: | ||
= A more realistic view of nuclear waste hazards = | = A more realistic view of nuclear waste hazards = | ||
{{Image|Dose Rate at Fuel Element Surface.png|right|350px|Fig.3 Dose Rate at Fuel Element Surface.}} | {{Image|Dose Rate at Fuel Element Surface.png|right|350px|Fig.3 Dose Rate at Fuel Element Surface.<ref>Graph is from Fig.4 in ''The Nuclear Waste Problem'', Jack Devanney (2022) [https://gordianknotbook.com]</ref>}} | ||
Figure 1 in the article exaggerates the long term danger of spent nuclear fuel. | Figure 1 in the [[Nuclear_waste_management]] article exaggerates the long term danger of spent nuclear fuel. | ||
Long term radioactivity is mostly alpha particles and electrons harmlessly trapped in the spent fuel. The more dangerous gamma rays die out in a few hundred years. | Long term radioactivity is mostly alpha particles and electrons (beta rays) harmlessly trapped in the spent fuel. The more dangerous high-energy photons (gamma rays) can pass through the fuel element metal casing, but they die out in a few hundred years. A better graph should show, not the total radioactivity inside the source, but the dose received by a person handling the spent fuel. Alpha particles can be stopped by a sheet of paper. Electrons are stopped by the outer layer of our skin. Instead of Becquerels per kilogram inside the source, we need dose rates at the fuel element surface. See Figure 3.<br> | ||
paper | |||
Dose is the amount of radiation energy absorbed by our tissue. Dose is measured in joules | Dose is the amount of radiation energy absorbed by our tissue. Dose is measured in joules | ||
per | per kilogram of tissue. Gray is the scientific name for joules per kg. Figure 3 is in milligrays per day | ||
(mGy/d). The key feature of Figure 3 is that photon decay is relatively rapid. By year 600, | (mGy/d). The key feature of Figure 3 is that photon decay is relatively rapid. By year 600, | ||
almost all the photon emitters are gone. In fact, the photon dose rate is so low that, according | almost all the photon emitters are gone. In fact, the photon dose rate is so low that, according | ||
to DOE rules, | to DOE rules, used fuel rods can be handled without any shielding at all. | ||
all. After year 600, the spent nuclear fuel must be swallowed in order to do any damage.<br> | After year 600, the spent nuclear fuel must be swallowed in order to do any damage.<br> | ||
Revision as of 05:11, 15 September 2022
A more realistic view of nuclear waste hazards
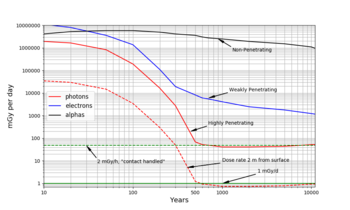
Figure 1 in the Nuclear_waste_management article exaggerates the long term danger of spent nuclear fuel.
Long term radioactivity is mostly alpha particles and electrons (beta rays) harmlessly trapped in the spent fuel. The more dangerous high-energy photons (gamma rays) can pass through the fuel element metal casing, but they die out in a few hundred years. A better graph should show, not the total radioactivity inside the source, but the dose received by a person handling the spent fuel. Alpha particles can be stopped by a sheet of paper. Electrons are stopped by the outer layer of our skin. Instead of Becquerels per kilogram inside the source, we need dose rates at the fuel element surface. See Figure 3.
Dose is the amount of radiation energy absorbed by our tissue. Dose is measured in joules
per kilogram of tissue. Gray is the scientific name for joules per kg. Figure 3 is in milligrays per day
(mGy/d). The key feature of Figure 3 is that photon decay is relatively rapid. By year 600,
almost all the photon emitters are gone. In fact, the photon dose rate is so low that, according
to DOE rules, used fuel rods can be handled without any shielding at all.
After year 600, the spent nuclear fuel must be swallowed in order to do any damage.