Tenofovir: Difference between revisions
imported>Caesar Schinas m (Bot: Replacing medical templates with CZMed) |
imported>Robert Badgett No edit summary |
||
| Line 32: | Line 32: | ||
* Apropovir | * Apropovir | ||
* Viread | * Viread | ||
==History== | |||
Viread brand of tenofovir was approved for Gilead Sciences by the [[Food and Drug Administration]] in the [[United States]] with a [http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/NewDrugApplicationNDA/ New Drug Application] (NDA) in 2001.<ref>{{FDA-Drug_Details|021356}}</ref> | |||
A generic version with a AB [[Food and Drug Administration/Catalogs/Therapeutic Equivalence Code|Therapeutic Equivalence Code]] was approved for Cipla Limited with a [http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ Abbreviated New Drug Application] (ANDA) in 2009.<ref>{{FDA-Drug_Details|078800}}</ref> The generic ANDA was reviewed under the expedited review provisions of the President’s Emergency Plan for AIDS Relief (PEPFAR) with the agreement that the drug would not be marketed in the United States until the Viread patent protection expires in 2017. | |||
==References== | |||
<references/> | |||
== External links == | == External links == | ||
{{CZMed}} | {{CZMed}} | ||
Revision as of 20:50, 29 January 2013
|
| |||||||
| tenofovir | |||||||
| |||||||
| Uses: | HIV/AIDS & Hep. B | ||||||
| Properties: | RT inhibitor, adenosine analog | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
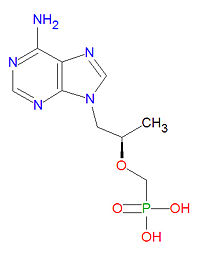
Tenofovir is a nucleotide analog reverse transcritase inhibitor (nRTI) antiviral drug used to treat HIV/AIDS and is in clinical trials for treatment of hepatitis B infection. The triphosphate from of the drug competes with the natural DNA nucleotide deoxyadenosine triphosphate (dATP) during DNA formation and it acts as a DNA chain terminator once incorporated because it lacks the normal deoxyribose sugar needed for connecting to the next DNA base.
Chemistry
The IUPAC chemical name for tenofovir is [(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethylphosphonic acid and it has chemical formula C9H14N5O4P, giving it a molecular mass of 287.2123 g/mol. It is chemically similar to the natural neucleotide adenosine but lacks a ribose sugar unit.
Synonyms and brand names
Synonyms
- Tenofovir disoproxil
- Tenofovir disoproxil fumarate
- D,L-Tenofovir
- TDF
- PMPA
Brand Names
- Apropovir
- Viread
History
Viread brand of tenofovir was approved for Gilead Sciences by the Food and Drug Administration in the United States with a New Drug Application (NDA) in 2001.[1]
A generic version with a AB Therapeutic Equivalence Code was approved for Cipla Limited with a Abbreviated New Drug Application (ANDA) in 2009.[2] The generic ANDA was reviewed under the expedited review provisions of the President’s Emergency Plan for AIDS Relief (PEPFAR) with the agreement that the drug would not be marketed in the United States until the Viread patent protection expires in 2017.
References
- ↑ Anonymous. Drugs@FDA for FDA Application No. 021356. U S Food and Drug Administration
- ↑ Anonymous. Drugs@FDA for FDA Application No. 078800. U S Food and Drug Administration
External links
The most up-to-date information about Tenofovir and other drugs can be found at the following sites.
- Tenofovir - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Tenofovir - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Tenofovir - Detailed information from DrugBank.