Search results
Jump to navigation
Jump to search
Page title matches
- |elName=Carbon '''Carbon''' is a [[Chemical elements|chemical element]], typically found as a [[Soli5 KB (806 words) - 17:16, 1 January 2021
- ...onfusing. Are we to assume diamond melts at 3550, but the resulting liquid carbon immediately resolidifies as graphite and then sublimes at 3800? Better stat391 bytes (56 words) - 05:06, 19 April 2011
- | pagename =Carbon | abc = Carbon2 KB (202 words) - 12:13, 20 July 2008
File:Carbon levels.png (1,962 × 2,556 (124 KB)) - 19:51, 11 March 2022- #REDIRECT[[Carbon capture and storage]]39 bytes (5 words) - 02:36, 8 May 2009
- 288 bytes (43 words) - 21:34, 6 November 2011
- 12 bytes (1 word) - 04:28, 9 November 2007
- 122 bytes (15 words) - 13:07, 7 July 2008
- ...gas that gives the sparkle to many soft drinks, some wines, and beer. The carbon dioxide gas freezes at −78.5 °C (−109.3 °F) and the frozen f835 bytes (138 words) - 21:20, 3 November 2011
File:Carbon with oxygen.JPG (409 × 286 (23 KB)) - 19:54, 11 March 2022- 12 bytes (1 word) - 12:13, 20 July 2008
- 12 bytes (1 word) - 15:00, 27 January 2008
File:Carbon configurations.jpg (296 × 274 (21 KB)) - 19:55, 11 March 2022File:Carbon atom.JPG (716 × 506 (99 KB)) - 19:57, 11 March 2022- #REDIRECT [[Carbon nanotube]]29 bytes (3 words) - 02:10, 12 October 2013
- 22 bytes (2 words) - 17:29, 27 August 2008
- '''Carbon nanotubes''' are tubular [[carbon]] molecules that have properties that make them potentially useful in [[nan A nanotube is a structure similar to a [[fullerene]], only the carbon [[atom]]s are rolled into a [[cylinder]] instead of a [[sphere]]; each end3 KB (427 words) - 10:31, 28 June 2023
- 4 bytes (0 words) - 23:25, 9 June 2008
- ...de is extremely toxic to humans and animals. Conversely, small amounts of carbon monoxide are produced in normal animal metabolism and it is thought to have ...ers. When combined with a metal (i.e., an [[organometallic]] complex), the carbon monoxide is a [[ligand]] called ''carbonyl'' : for example, in nicke17 KB (2,453 words) - 09:37, 6 March 2024
- 12 bytes (1 word) - 02:33, 12 October 2013
- #REDIRECT [[Carbon/Periodic table]]35 bytes (4 words) - 07:05, 6 March 2024
- | pagename = Carbon monoxide | abc = Carbon monoxide2 KB (328 words) - 21:27, 6 November 2011
- 1 bytes (1 word) - 17:53, 10 June 2008
- Chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom.125 bytes (17 words) - 09:13, 27 January 2009
- ...teless [[gas]] that is slightly lighter than [[air]] and consists of one [[carbon]] [[atom]] and one [[oxygen]] atom.183 bytes (25 words) - 21:30, 6 November 2011
- <includeonly>Non-Metal</includeonly><noinclude>Carbon is a [[Non-Metal]].</noinclude>85 bytes (9 words) - 07:07, 6 March 2024
- 12 bytes (1 word) - 02:33, 12 October 2013
File:Carbon electron configuration.JPG (692 × 170 (28 KB)) - 19:53, 11 March 2022- <noinclude>Carbon has many allotropes. For more information see: [[Carbon/Phase diagram]]</noinclude>159 bytes (18 words) - 13:12, 17 April 2011
- #REDIRECT[[Carbon capture and storage]]39 bytes (5 words) - 02:44, 8 May 2009
- 12 bytes (1 word) - 21:44, 6 November 2011
- 805 bytes (105 words) - 06:58, 6 March 2024
- 487 bytes (78 words) - 12:02, 9 June 2009
- Allotropes of carbon with an extremely thin, hollow cylindrical nanostructure.114 bytes (13 words) - 02:47, 12 October 2013
- 34 bytes (7 words) - 18:03, 27 July 2008
- | pagename = Carbon dioxide | abc = Carbon dioxide705 bytes (60 words) - 06:03, 15 March 2024
- 60 bytes (7 words) - 13:08, 17 April 2011
- 264 bytes (35 words) - 16:26, 17 May 2010

File:Carbon bohr model.gif (314 × 359 (26 KB)) - 19:58, 11 March 2022- 12 bytes (1 word) - 15:01, 27 January 2008
- 1 bytes (0 words) - 23:24, 9 June 2008
- #REDIRECT [[Carbon nanotube/Definition]]40 bytes (4 words) - 02:10, 12 October 2013
- 43 bytes (5 words) - 04:31, 6 June 2009
- | pagename = Carbon nanotube | abc = Carbon nanotube828 bytes (67 words) - 02:32, 12 October 2013
- 505 bytes (80 words) - 03:59, 6 June 2009
- 275 bytes (35 words) - 05:42, 6 March 2024
- 5 bytes (1 word) - 13:32, 10 June 2008
- 50 bytes (7 words) - 04:36, 6 June 2009
- 827 bytes (133 words) - 02:33, 12 October 2013
- ...concept and as yet (2007) no large scale power plant operates with a full carbon capture and storage system. ...l be more expensive. Rarely considered in such mathematics is the embodied carbon cost of the equipment and machinery needed to create this act of reverse-en33 KB (5,096 words) - 06:33, 10 October 2013
- 12 bytes (1 word) - 02:33, 12 October 2013
- #REDIRECT[[Carbon capture and storage]]39 bytes (5 words) - 02:42, 8 May 2009
- 1 KB (170 words) - 17:22, 8 May 2009
- {{r|Carbon dioxide}}288 bytes (34 words) - 21:52, 6 November 2011

File:Low-carbon-share-energy1.png |description = Share of energy from low-carbon sources |source = https://ourworldindata.org/grapher/low-carbon-share-energy?tab=chart&country=ITA~OWID_WRL~FRA~CHN~IND~JPN~USA~SWE~ARE(3,400 × 2,400 (671 KB)) - 16:16, 3 June 2022- Auto-populated based on [[Special:WhatLinksHere/Carbon dioxide]]. Needs checking by a human. {{r|Carbon capture and storage}}3 KB (351 words) - 21:51, 16 August 2010
- 12 bytes (1 word) - 21:45, 6 November 2011

File:Carbon Intensity FH9oR-ZXsAkcHMn.png (1,200 × 787 (647 KB)) - 07:24, 20 February 2023- A carbon fixation reaction that fixes carbon dioxide into the four carbon molecule oxaloacetate; usually found in the mesophyll cells of plants that213 bytes (30 words) - 16:14, 17 May 2010
- | pagename = Carbon capture and storage | abc = carbon capture and storage836 bytes (75 words) - 19:21, 13 June 2010
- 551 bytes (85 words) - 05:15, 23 October 2013
- An approach to reduce emissions of greenhouse gases by capturing carbon dioxide (CO<sub>2</sub>).133 bytes (18 words) - 02:56, 8 May 2009
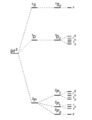
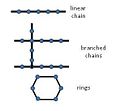
- ...itself facilitates assembly of long chains and rings of carbon; and small carbon molecules (like [[sugar]]s, [[amino acid]]s and [[nucleotide]]s) easily joi ...e combinations. The shapes of the bonding orbitals of at least some of the carbon bonds add yet additional levels of information -for example, in double bond3 KB (504 words) - 18:45, 7 June 2007
- ...rccs.htm Intergovernmental Panel on Climate Change] IPCC Special Report on Carbon Dioxide Capture and Storage. *[http://arstechnica.com/journals/science.ars/2006/9/19/5341 Carbon Dioxide Lakes in the Deep Ocean] Posted by John Timmer, September 20062 KB (330 words) - 15:03, 8 May 2009

File:Build-times of low-carbon electricity.png (732 × 732 (272 KB)) - 14:57, 20 June 2022- 644 bytes (92 words) - 14:33, 8 May 2009

File:Materials Requirements for Low Carbon Power.png (2,260 × 1,194 (229 KB)) - 17:11, 4 December 2022
File:Carbon Intensity 331896563 761120832401090 2334271138528851346 n.jpg (960 × 540 (81 KB)) - 07:15, 20 February 2023- ...itself facilitates assembly of long chains and rings of carbon; and small carbon molecules (like [[sugar]]s, [[amino acid]]s and [[nucleotide]]s) easily joi ...e combinations. The shapes of the bonding orbitals of at least some of the carbon bonds add yet additional levels of information -for example, in double bond3 KB (469 words) - 18:44, 7 June 2007
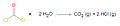
File:Decomposition of phosgene to hydrochloric acid and carbon dioxide.jpg (600 × 130 (49 KB)) - 19:54, 11 March 2022- 37 bytes (6 words) - 03:56, 6 June 2009
Page text matches
- A carbon fixation reaction that fixes carbon dioxide into the four carbon molecule oxaloacetate; usually found in the mesophyll cells of plants that213 bytes (30 words) - 16:14, 17 May 2010
- <noinclude>Carbon has many allotropes. For more information see: [[Carbon/Phase diagram]]</noinclude>159 bytes (18 words) - 13:12, 17 April 2011
- ...nd with one connected carbon atom and a single bond with another connected carbon; the molecule may have more than one ring and many side chains280 bytes (51 words) - 18:57, 21 April 2010
- A stable allotrope of carbon where the carbon atoms are arranged in an isometric-hexoctahedral crystal lattice, commonly202 bytes (26 words) - 09:42, 24 January 2021
- == Charcoal an allotrope of carbon? == ...than its arrangement of carbon atoms. The [[fullerenes]] are allotropes of carbon, though, and deserve a mention.1 KB (177 words) - 14:55, 1 February 2010
- ...red for the first step of the Calvin cycle to reduce carbon dioxide during carbon fixation.231 bytes (32 words) - 16:31, 17 May 2010
- ...arbon/fibre epoxy composites. Commercilization of high Modulus Pitch based carbon fibres with a focus on industrial applications. Education: PhD. Composite M467 bytes (58 words) - 03:36, 22 November 2023
- |[[Carbon]]: | [[Carbon/Boiling point|{{:Carbon/Boiling point}}]]1 KB (141 words) - 12:54, 26 April 2009
- |[[Carbon]]: | {{:Carbon/Boiling point}}1 KB (158 words) - 18:09, 23 June 2008
- ...rccs.htm Intergovernmental Panel on Climate Change] IPCC Special Report on Carbon Dioxide Capture and Storage. *[http://arstechnica.com/journals/science.ars/2006/9/19/5341 Carbon Dioxide Lakes in the Deep Ocean] Posted by John Timmer, September 20062 KB (330 words) - 15:03, 8 May 2009
- ...esonance imaging]] (MRI) and [[nuclear magnetic resonance]] spectroscopy. Carbon-14 is radioactive, and is therefore useful for radiation tracing and [[Carb1 KB (147 words) - 16:35, 16 January 2022
- ...lements|chemical element]], [[carbon]]. It is the most stable allotrope of carbon in [[International Union of Pure and Applied Chemistry]] prescribed [[stand Graphite is one of two [[crystallographic]] carbon allotropes, the other being [[diamond]]. Graphite has a [[lamellar]] struct596 bytes (68 words) - 22:39, 22 October 2010
- ...ole as malic acid until the following light period when it is converted to carbon dioxide for fixation by the Calvin cycle.302 bytes (50 words) - 16:22, 17 May 2010
- ...gas that gives the sparkle to many soft drinks, some wines, and beer. The carbon dioxide gas freezes at −78.5 °C (−109.3 °F) and the frozen f835 bytes (138 words) - 21:20, 3 November 2011
- #REDIRECT [[Carbon dioxide]]28 bytes (3 words) - 05:37, 11 November 2007
- #REDIRECT [[Carbon nanotube]]29 bytes (3 words) - 02:10, 12 October 2013
- {{r|Carbon dioxide}} {{r|Carbon monoxide}}373 bytes (47 words) - 02:53, 26 May 2010
- #REDIRECT [[Carbon nanotube/Definition]]40 bytes (4 words) - 02:10, 12 October 2013
- #REDIRECT [[Carbon/Periodic table]]35 bytes (4 words) - 07:05, 6 March 2024
- ...nd [[chemical pneumonitis]]. Phosgene has many different names, including carbon oxychloride, chloroformyl chloride, carbonyl chloride, carbonic dichloride, ...water molecules act as [[nucleophile|nucleophiles]] and attack the central carbon atom.1 KB (194 words) - 12:46, 11 June 2009
- #REDIRECT[[Carbon capture and storage]]39 bytes (5 words) - 02:36, 8 May 2009
- #REDIRECT[[Carbon capture and storage]]39 bytes (5 words) - 02:42, 8 May 2009
- #REDIRECT[[Carbon capture and storage]]39 bytes (5 words) - 16:39, 2 September 2009
- #REDIRECT[[Carbon capture and storage]]39 bytes (5 words) - 02:44, 8 May 2009
- #REDIRECT[[Carbon capture and storage]]39 bytes (5 words) - 02:45, 8 May 2009
- A '''carbonyl group''' is a [[functional group]] which consists of a [[carbon]] atom with is double bound to an [[oxygen]] atom. Closely related chemica ...s [[electrophile|electrophilic]]. As such, [[nucleophilic attack]] of the carbon atom is a useful reaction mechanism for chemicals containing a carbonyl gro727 bytes (121 words) - 08:16, 24 September 2008
- ...Environmental Audit Committee Inquiry into: Carbon Capture and Storage] [[Carbon Capture and Storage Association]] (CCSA) , London, England807 bytes (105 words) - 22:45, 4 March 2009
- ...they are called a [[furanose]]. When fructose cyclizes into such a five carbon ring, it is called [[fructofuranose]].862 bytes (124 words) - 15:09, 6 February 2008
- ...ion]], that is, by conversion of all unsaturated (double and triple carbon-carbon) bonds to saturated (single) C—C bonds by addition of [[hydrogen]].813 bytes (121 words) - 07:57, 13 August 2009
- ...he main greenhouse gases for Earth are [[water]] vapor (H<sub>2</sub>O), [[carbon dioxide]] (CO<sub>2</sub>), and [[ozone]] (O<sub>3</sub>). Some other gases ...bon dioxide emissions. See [[Kyoto Protocol]], [[emissions trading]] and [[carbon credits]].805 bytes (117 words) - 14:06, 26 January 2009
- Aromatic molecules which contain atoms other than carbon in the ring.106 bytes (14 words) - 01:44, 3 November 2010
- | pagename = Carbon dioxide | abc = Carbon dioxide705 bytes (60 words) - 06:03, 15 March 2024
- A naturally occurring allotrope of the element carbon.91 bytes (11 words) - 10:19, 9 October 2010
- A class of molecules that contain only [[carbon]] and [[hydrogen]] atoms.110 bytes (14 words) - 19:37, 22 March 2009
- An organic compound containing a carbon triple bonded to nitrogen.102 bytes (13 words) - 20:19, 29 November 2009
- Allotropes of carbon with an extremely thin, hollow cylindrical nanostructure.114 bytes (13 words) - 02:47, 12 October 2013
- A monosaccharide with six carbon atoms, having the chemical formula C6H12O6.113 bytes (16 words) - 20:23, 3 September 2009
- | pagename = Carbon nanotube | abc = Carbon nanotube828 bytes (67 words) - 02:32, 12 October 2013
- '''Capnography''' is a technique for continuously monitoring the [[carbon dioxide]] content of expired air. It is particularly useful in field and em ...[[blood gas analysis]] by focusing on the actual gas exchange rather than carbon dioxide in blood. Like [[pulse oximetry]], it is a relatively noninvasive t507 bytes (69 words) - 10:23, 22 June 2010
- A decreased [[partial pressure]] of [[carbon dioxide]] in the [[blood]].108 bytes (13 words) - 17:59, 25 May 2010
- An increased [[partial pressure]] of [[carbon dioxide]] in the [[blood]].109 bytes (13 words) - 17:58, 25 May 2010
- An organic molecule that contains exclusively carbon and hydrogen atoms, with only single bonds between carbons147 bytes (19 words) - 15:11, 5 February 2009
- | pagename = Carbon capture and storage | abc = carbon capture and storage836 bytes (75 words) - 19:21, 13 June 2010
- ...itself facilitates assembly of long chains and rings of carbon; and small carbon molecules (like [[sugar]]s, [[amino acid]]s and [[nucleotide]]s) easily joi ...e combinations. The shapes of the bonding orbitals of at least some of the carbon bonds add yet additional levels of information -for example, in double bond3 KB (469 words) - 18:44, 7 June 2007
- An approach to reduce emissions of greenhouse gases by capturing carbon dioxide (CO<sub>2</sub>).133 bytes (18 words) - 02:56, 8 May 2009
- Chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom.125 bytes (17 words) - 09:13, 27 January 2009
- A type or class of sex [[steroid]] [[hormone]] with an eighteen carbon atom base structure.127 bytes (18 words) - 20:58, 6 April 2009
- [[Chemical compound]]s containing [[carbon]]-[[silicon]] [[chemical bond|bonds]].117 bytes (13 words) - 16:06, 13 December 2008
- *[[Carbon]]215 bytes (17 words) - 09:15, 6 March 2024
- Continuous recording of the carbon dioxide content of expired air.<noinclude>{{DefMeSH}}</noinclude>136 bytes (16 words) - 18:30, 25 May 2010
- A drawing technique that uses soft, black or dark gray, carbon to draw lines and shading on textured paper142 bytes (22 words) - 18:20, 16 January 2009
- ...itself facilitates assembly of long chains and rings of carbon; and small carbon molecules (like [[sugar]]s, [[amino acid]]s and [[nucleotide]]s) easily joi ...e combinations. The shapes of the bonding orbitals of at least some of the carbon bonds add yet additional levels of information -for example, in double bond3 KB (504 words) - 18:45, 7 June 2007
- A type or class of sex [[steroid]] [[hormone]] with a nineteen carbon atom base structure.126 bytes (18 words) - 17:29, 31 May 2009
- ...] gas as a coolant, that might provide process heat for production of zero-carbon [[hydrogen]] from [[water]].<ref>https://www.gen-4.org/gif/jcms/c_9362/vhtr196 bytes (31 words) - 02:53, 7 April 2024
- Common name for [[sodium bicarbonate]]; usage comes from its generation of [[carbon dioxide]] when subjected to heat or acid; generate gas leavens a baked prod198 bytes (28 words) - 21:03, 10 October 2010
- ...ible material releasing heat, light, and various reaction products such as carbon dioxide and water.169 bytes (23 words) - 10:48, 3 September 2009
- An [[alloy]] whose major component is [[iron]], with [[carbon]] content between 0.02% and 1.7% by weight, depending on [[grade (steel)|gr179 bytes (22 words) - 10:07, 15 March 2010
- A chemical group containing a carbon atom double bonded to an oxygen atom.110 bytes (16 words) - 08:02, 24 September 2008
- {{Image|Carbon Intensity FH9oR-ZXsAkcHMn.png|right|350px|Add image caption here.}} {{Image|Build-times of low-carbon electricity.png|right|350px|How fast can we get off fossil fuels, if we dec576 bytes (78 words) - 17:52, 15 March 2024
- ...tions of [[amine]]s to remove [[hydrogen sulphide]] (H<sub>2</sub>S) and [[carbon dioxide]] (CO<sub>2</sub>) from [[gas]]es187 bytes (28 words) - 09:37, 6 March 2024
- The means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and propagate.143 bytes (23 words) - 09:14, 16 June 2008
- ...and chemist, known for his discoveries of latent heat, specific heat, and carbon dioxide159 bytes (19 words) - 03:26, 21 May 2008
- An omega-3 fatty acid consisting of a carboxylic acid with a 22-carbon chain, and six cis double bonds.140 bytes (21 words) - 10:07, 3 September 2009
- A molecule containing only carbon and hydrogen that exhibits unusual stability and reactivity from having a c202 bytes (25 words) - 17:38, 2 November 2010
- A six carbon aromatic compound commonly used in industry as a precursor for other import172 bytes (25 words) - 02:16, 28 April 2009
- |elName=Carbon '''Carbon''' is a [[Chemical elements|chemical element]], typically found as a [[Soli5 KB (806 words) - 17:16, 1 January 2021
- ...c method of determining the age of organic material based on the amount of carbon-14.131 bytes (19 words) - 15:46, 24 September 2012
- ...with short-lived [[positron]]-emitting [[isotope|radionuclides]] (such as carbon-11, nitrogen-13, oxygen-15 and fluorine-18) to measure cell metabolism.<noi279 bytes (30 words) - 02:19, 15 May 2010
- ...cle, discovered by Melvin Calvin, that is responsible for the reduction of carbon dioxide to sugar in the stroma of chloroplasts.179 bytes (26 words) - 16:04, 17 May 2010
- The matrix of a plastid that contains the enzymes for carbon fixation and the organelles DNA; thylakoid membranes are surrounded by this180 bytes (26 words) - 15:56, 17 May 2010
- ...p, a carboxyl group, a hydrogen atom, and a side chain bonded to a central carbon.148 bytes (23 words) - 20:58, 5 October 2009
- <includeonly>Non-Metal</includeonly><noinclude>Carbon is a [[Non-Metal]].</noinclude>85 bytes (9 words) - 07:07, 6 March 2024
- ...[yeast]] that [[Fermentation (food)|ferment]] sugar into [[ethanol]] and [[carbon dioxide]].206 bytes (26 words) - 05:23, 1 January 2008
- ...e of the [[pH]] of the oceans, presumably due to the increased intake of [[carbon dioxide]] from the [[atmosphere]].159 bytes (22 words) - 00:13, 14 July 2008
- ...teless [[gas]] that is slightly lighter than [[air]] and consists of one [[carbon]] [[atom]] and one [[oxygen]] atom.183 bytes (25 words) - 21:30, 6 November 2011
- ...into its system. As carbon-14 stops being absorbed when an organism dies. Carbon-14 is a [[radioactivity|radioactive]] [[isotope]] with a [[half-life]] of 5 ...l Prize in Chemistry|Nobel Prize]] for his research.<ref name=KG2002-161/> Carbon-14 has a nucleus of six [[proton]]s and eight [[neutron]]s, making it unsta2 KB (367 words) - 15:47, 24 September 2012
- ...s the omega carbon, the first double bond occurs at carbon 20, the omega-3 carbon. Like other omega-3 fatty acids, it can be found in fish. Most of the DHA2 KB (224 words) - 11:27, 15 September 2013
- ...ntake of [[carbon dioxide]] from the [[atmosphere]]. Due to the release of carbon dioxide by human activity, the increased [[acid]]ity of the oceans could ca [[Carbon dioxide]] (CO<sub>2</sub>) combines with [[water]] (H<sub>2</sub>O) to form2 KB (371 words) - 01:49, 9 March 2008
- One twelfth of the mass of a carbon-12 atom in its nuclear and electronic ground state. It is equal to the unif169 bytes (28 words) - 00:10, 2 May 2009
- Wallace, A, ''Technocracy: Building a new sustainable society for a post carbon world'' Network of European Technocrats. 2007. ISBN 978-9-1633-1249-6221 bytes (34 words) - 22:10, 7 September 2008
- The number of atoms in 12 gram of carbon-12 atoms in their ground state at rest.117 bytes (18 words) - 11:00, 24 June 2009
- (G6P), is glucose that has been phosphorylated on carbon 6. The conversion from glucose to G6P is the first step of glycolysis for e194 bytes (31 words) - 15:11, 1 February 2009
File:Large grids low carbon.png |description = large electrical grids with very low carbon emissions(1,496 × 1,198 (824 KB)) - 06:24, 27 November 2022- '''Coke''' is a manmade fuel, consisting of almost pure carbon.<ref name=EiaCokeDef/> ...leum]]. The feed stock is heated in air-tight containers, to separate the carbon from the volatile elements.3 KB (295 words) - 15:16, 21 January 2024
- ...ioxide]] (CO<sub>2</sub>), hydrogen sulfide or [[mercaptan]]s (RSH). Thus, carbon dioxide by itself is an acid gas but not a sour gas. Before a raw natural gas containing hydrogen sulfide or carbon dioxide can be used, the raw gas must be treated to reduce those impurities2 KB (360 words) - 08:07, 15 March 2024
- ...strength as well as in 3-D conformation - but are all remarkably stable. Carbon atoms easily join into longer chains and closed rings; and the small organi ...e three entirely different geometries. Changing from one type of carbon-to-carbon bond to another type, as when a double bond is reduced to a single bond, wi4 KB (625 words) - 06:28, 6 March 2024
- ...the ATP and NADPH synthesized during the light-dependent reactions to fix carbon atoms from CO<sub>2</sub>.236 bytes (34 words) - 00:33, 8 January 2010
- ...ic number of [[neutron]]s and hence a specific [[nuclear mass]], such as [[carbon]]-14 (<sup>14</sup>C).191 bytes (29 words) - 10:42, 6 July 2008
- ...eral factors that influence their properties such as shape, arrangement of carbon atoms,and whether the CNTs are single-walled or multi-walled [http://www.pe As a result of the strong carbon-to-carbon bonding, CNTs exhibit unique properties in material sciences and electronic7 KB (1,012 words) - 03:48, 22 November 2023
- ...nd utility. They are naturally formed from amounts of highly compressed [[carbon]] found below into the Earth's [[mantle (geology)|mantle]] (roughly 160 km ...[[valence shell]]), shares all of its valence electrons with a neighboring carbon atom, forming a structure called a [[covalent bond]]. Trace elements may b3 KB (442 words) - 10:07, 28 February 2024
- Test which measures the amounts of oxygen and carbon dioxide in the blood, as well as the acidity (pH) of the blood.153 bytes (25 words) - 02:43, 5 September 2009
- [[Organic compound]]s containing [[carbon]], [[hydrogen]], and [[oxygen]]; includes [[sugar]]s and [[starch]]es that280 bytes (38 words) - 07:50, 7 April 2010
- ...and preparation (by synthesis or by other means) of chemical compounds of carbon and hydrogen, which may contain any number of other elements.253 bytes (35 words) - 17:12, 13 May 2008
- Oxidative degradation of saturated fatty acids in which two-carbon units are sequentially removed from the molecule with each turn of the cycl266 bytes (41 words) - 09:30, 5 September 2009
- ...macrocycle consists of four pyrrole-type units interconnected at their α carbon atoms via a methine bridge, and functioning as a metal-binding cofactor in286 bytes (38 words) - 06:15, 6 September 2009
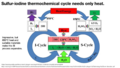
File:Sulfur-Iodine Process.png |description = Sulfur-Iodine Process to make carbon-free hydrogen from water(1,600 × 900 (703 KB)) - 12:56, 19 May 2022
File:Low-carbon-share-energy1.png |description = Share of energy from low-carbon sources |source = https://ourworldindata.org/grapher/low-carbon-share-energy?tab=chart&country=ITA~OWID_WRL~FRA~CHN~IND~JPN~USA~SWE~ARE(3,400 × 2,400 (671 KB)) - 16:16, 3 June 2022- ...ndamentals] (written by Paul Ellis and Christopher Paul of the Great Lakes Carbon Corporation)360 bytes (51 words) - 15:54, 1 April 2008
- ...chemical compound]] with the [[chemical formula|formula]] [[Silicon|Si]]([[Carbon|C]][[Hydrogen|H]]<sub>3</sub>)<sub>4</sub>, used as a standard in <sup>1</s308 bytes (45 words) - 06:39, 7 April 2010
- ...ses such as [[carbon dioxide]] (CO<sub>2</sub>) or hydrogen sulfide. Thus, carbon dioxide by itself is an acid gas but it is not a sour gas. Before a raw natural gas containing hydrogen sulfide and/or carbon dioxide can be used, the raw gas must be treated to remove those impurities3 KB (430 words) - 09:44, 6 March 2024

