Pyrroloquinoline quinone: Difference between revisions
imported>Pedro Silva No edit summary |
imported>Caesar Schinas m (Bot: Delinking years) |
||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | |||
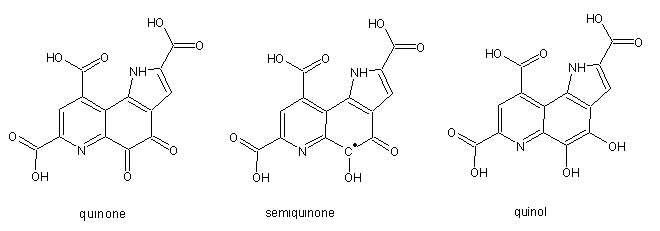
PQQ was reported to be a [[vitamin]] in [[mouse|mice]] on | '''Pyrroloquinoline quinone''' (PQQ) was originally discovered in 1978 in methanol dehydrogenase fom <i>Hyphomicrobium</i> X. It is present as a non-covalently bound [[cofactor]] in several bacterial [[dehydrogenase]]s involved in the oxidation of alcohols and sugars. The biochemically relevant redox states are the fully oxidized quinone form, the radical semiquinone form and the reduced quinol. The reduced form is only stable when bound to enzyme or at low pH, whereas the semiquinone is only stable at high pH and anerobic conditions. | ||
[[Image:Pqq.GIF]]<br> | |||
The pKa values of the protonable groups in PQQ are: | |||
* 0.3 (pyridyl nitrogen) | |||
* 1.6 and 2.2 for the carboxylic groups in the pyridine ring | |||
* 3.3 for the carboxyl group in the pyrrole ring | |||
* 10.3 for the pyrrole nitrogen | |||
PQQ was reported to be a [[vitamin]] in [[mouse|mice]] on April 24, 2003 by a research team led by Takafumi Kato of the [http://www.riken.go.jp/engn/index.html Japanese Institute of Physical and Chemical Research] ([http://www.brain.riken.jp/labs/mdmd/pqq/ source]). However, these conclusions have been strongly challenged. | |||
==References== | ==References== | ||
* Role of PQQ as a mammalian enzyme cofactor? [ | * [http://dx.doi.org/10.1038/nature03322 Leigh M. Felton and C. Anthony, Role of PQQ as a mammalian enzyme cofactor? ] | ||
* [http://dx.doi.org/10.1038/nature03323 Robert Rucker, David Storms, Annemarie Sheets, Eskouhie Tchaparian,Andrea Fascetti Is pyrroloquinoline quinone a vitamin? ] | |||
Latest revision as of 07:10, 9 June 2009
Pyrroloquinoline quinone (PQQ) was originally discovered in 1978 in methanol dehydrogenase fom Hyphomicrobium X. It is present as a non-covalently bound cofactor in several bacterial dehydrogenases involved in the oxidation of alcohols and sugars. The biochemically relevant redox states are the fully oxidized quinone form, the radical semiquinone form and the reduced quinol. The reduced form is only stable when bound to enzyme or at low pH, whereas the semiquinone is only stable at high pH and anerobic conditions.
The pKa values of the protonable groups in PQQ are:
- 0.3 (pyridyl nitrogen)
- 1.6 and 2.2 for the carboxylic groups in the pyridine ring
- 3.3 for the carboxyl group in the pyrrole ring
- 10.3 for the pyrrole nitrogen
PQQ was reported to be a vitamin in mice on April 24, 2003 by a research team led by Takafumi Kato of the Japanese Institute of Physical and Chemical Research (source). However, these conclusions have been strongly challenged.