Particle in a box: Difference between revisions
imported>Michael Underwood m (→Alternate description: Added mention that the energy eigenvalues are not affected by the translation) |
imported>John Stephenson (categories) |
||
| Line 97: | Line 97: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
[[Category: Physics Workgroup]] | |||
[[Category: CZ Live]] | |||
Revision as of 00:08, 23 June 2007
The particle in a box or infinite square well problem is one of the simplest non-trivial solutions to Schrödinger's wave equation. As such it is often encountered in introductory quantum mechanics material as a demonstration of the quantization of energy. In its simplest form the problem is one-dimensional, and involves a single particle living in an infinite potential well.
The 1D square well
Setting up the problem
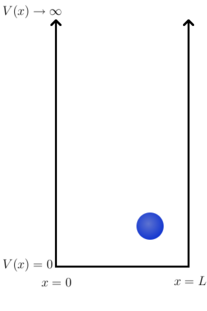
The potential can be given by
- for and otherwise.
That is, in a region of length (the box or well) the potential is zero; everywhere else it is infinite. An immediate consequence of this is that the particle must be located somewhere between and , since if it were anywhere else it would have to have infinite energy.
Since does not depend on time we can use the time-independent version of the Schrödinger equation,
where is Planck's constant divided by 2π, and and are the mass and energy of the particle, respectively. This is nothing but the eigenvalue problem, and our task is to determine the energy eigenvalue(s) and eigenstate(s) that solve it. The equation is a second-order linear ODE with constant coefficients, and the general solution can be written as
where and are complex constants to be determined from conditions on the system. The first condition we can use is that the wavefunction must be continuous. We know that the particle cannot exist at positions , which tells us that the wavefunction must be identically zero there (in order for the probability of finding the particle there to also be zero). Continuity then implies that at the wavefunction is also zero, so we have
meaning that the wavefunction can now be written
Energy quantization
Using the same continuity argument at tells us that
One solution to this is of course . However, this would mean that the wavefunction vanishes everywhere -- implying that there is no particle! The other way to satisfy this equality is to have the sine term vanish, which will happen if
where mathematically can be any integer. This problem is not purely mathematical though, and we know for physical reasons that , , , and are all greater than zero. This means that must also be greater than zero, so is restricted to being a natural number, . We can now solve for the particle's energy,
where we have labelled the energy by the integer . We have just derived energy quantization! Without the potential well, i.e. if the particle was free, its energy would be allowed to take on any real number. Once inside the box though, only a specific discrete set of energy eigenvalues is permitted.
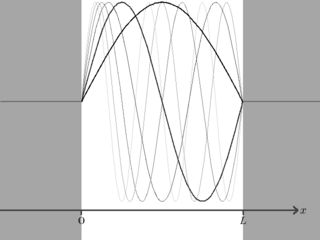
Substituting back into , we now have an infinite number of possible wavefunctions for the particle,
The final step is to determine the value of . This requires another condition that we can impose upon the problem.
Normalization
The probability of finding the particle somewhere must be . Since the wavefunction vanishes everywhere outside , the normalization condition is
Solving for tells us , where is some overall quantum phase (a real number). Because overall phases do not affect measurable results we are free to choose any value we want for without affecting the physics. For simplicity then, we set .
The solutions
We have now solved the particle-in-a-box problem. According to the Schrödinger wave equation the particle confined to the (infinite) box can only take on certain energy eigenvalues, namely
and the wavefunction for the particle when it is in the th energy eigenstate is
inside the box, and outside it.
Alternate description
Another common (equivalent) way to describe this problem is to shift the location of the box, so that it runs from to instead of to . The derivation of the allowed wavefunctions is very similar to what we did here, or the results can be obtained via coordinate transformation (specifically, a translation). With this different description of the square well the energy eigenvalues remain unchanged (as is to be expected[1]) while the resulting wavefunctions are given by[2]
if is odd, and
if is even.
Properties / Discussion / Comments (under construction)
The are complete.
is not allowed because implies wavefunction vanishes everywhere; no particle. So lowest energy state is above zero.
As energy spacing goes to zero; recover the free space result (especially in the Alternate description version...).
Generalization to 3D (to be done)
Spherical well
Cubic well
References
- ↑ The energy of a particle should not depend in any way on our choice of coordinate system, i.e. where we choose to place the origin .
- ↑ A. Messiah (1999) Quantum Mechanics: Two volumes bound as one ISBN 0486409244




























![{\displaystyle [0,L]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/53e3bfad6c2b8297e8c8d2e84ce8f869d69b0d86)