Organosilicon
Organosilicon compounds are chemical compounds containing carbon silicon bonds. Organosilicon chemistry is the corresponding science exploring the properties and reactivity of organosilicon compounds [1]. Most of organosilicon compounds are tetravalent and tetrahedral. Hypervalent silicon is rare, and occurs mainly in organosilicon complexes obtained in non-aqueous solutions, in some glasses, and at high pressures within the earth in dense phases such as stishovite. [2] In aqueous solution, the hexaoxo-center is documented only for complexes in which silicon is chelated by catechol (yielding tris[1,2-benzenediolato]silicate) [3] Cite error: Closing </ref> missing for <ref> tag, 2-hydroxypyridine N-oxide [4], tropolone [5], or closely related analogs; pentaoxosilicon is unknown until recently. [6]
Unlike carbon, silicon is not found in any biomolecules [7]. However, it was found recently that many sugars could form stable pentavalent and/or hexavalent complexes with silicate in aqueous solution under ambient conditions. [8] This study suggested that silicates might be extensively involved in the prebiotic chemistry.
The first organosilicon compound, tetraethylsilane was discovered by Charles Friedel and James Crafts in 1863 by reaction of tetrachlorosilane with diethyl zinc. Discovered in 1893, the simplest marriage between silicon and carbon is Silicon carbide which has many industrial applications.
Organosilanes
Carbon silicon bonds compared to carbon carbon bonds are longer (186 pm vs. 154 pm) and weaker with bond dissociation energy 451 KJ/mole vs 607 KJ/mol [9]. The C-Si is strongly polarized towards carbon due to its higher electronegativity ( C 2.55 vs Si 1.90). One manifestation of bond polarization in organosilanes is found in the Sakurai reaction.
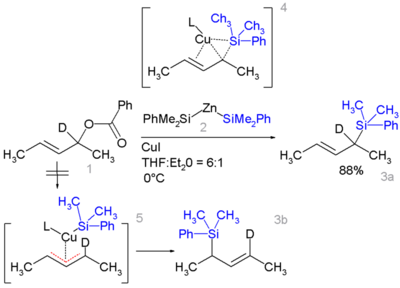
Certain allyl silanes can be prepared from allylic ester such as 1 and monosilylcopper compounds such as 2 in [10] [11].
In this reaction type silicon polarity is reversed in a chemical bond with zinc and a formal allylic substitution on the benzoyloxy group takes place.
The chemistry of silanes such as tetramethylsilane is comparable to that of alkanes in many aspects such as thermal stability. The β-silicon effect describes the stabilizing effect of a β-silicon atom on a carbocation with many implications for reactivity.
Siloxides
More notably bonds of silicon to oxygen are much shorter and stronger (809 compared to 538 KJ/mole) than that of those of carbon to oxygen. The polarization in this bond increases towards oxygen. Examples are the Siloxanes and the polymeric polysiloxanes. Silyl ethers are extensively used as protective groups for alcohols. Only silicon bonds to fluorine are stronger and that is why the fluorine source TASF (or more commonly TBAF) is useful in deprotection. The favorable formation of Si-O bonds drive many organic reaction such as the Brook rearrangement and Peterson olefination.
Silyl hydrides
The silicon to hydrogen bond is longer than the C-H bond (105 compared to 148 pm) and weaker (338 compared to 299 KJ/mole). Hydrogen is more electronegative than silicon hence the naming convention of silyl hydrides. Silyl hydrides are very reactive and used as reducing agents for example PMHS.
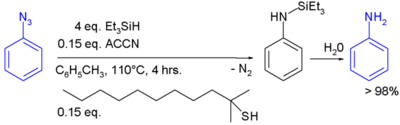
In one study triethylsilylhydride is used in the conversion of an phenyl azide to an aniline [12]:
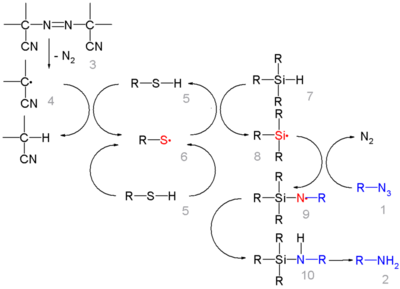
In this reaction ACCN is a radical initiator and an aliphatic thiol transfers radical character to the silylhydride. The triethylsilyl free radical then reacts with the azide with expusion of nitrogen to a N-silylarylaminyl radical which grabs a proton from a thiol completing the catalytic cycle:
Aqueous workup then gives aniline.
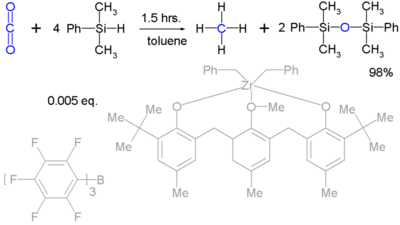
Silyl hydrides can even take up the reduction of robust molecules such as carbon dioxide (to methane) [13]:
Although it takes a very complex catalyst system.
Silenes
Organosilicon compounds unlike their carbon counterparts do not have a rich double bond chemistry due to the large difference in electronegativity. Existing compounds with organosilene Si=C bonds are laboratory curiosities such as the silicon benzene analogue silabenzene, and Si=Si bond containing disilenes.
See also
- Compounds of carbon with period 3 elements: organosilicon compounds, organophosphorus compounds, organosulfur compounds,
- Compounds of carbon with other group 14 elements: organosilicon compounds, organogermanium compounds, organotin compounds, organolead compounds.
- silylenes, the carbene counterparts and silylenoids the carbenoid counterparts.
External links
- organosilicon chemistry @ http://users.ox.ac.uk Link
References
- ↑ Silicon in Organic Synthesis Colvin, E. Butterworth: London 1981
- ↑ Herreros B. et al., Science, 1994, 263, 1585-1587.
- ↑ A. Rosenheim, B. Reibmann, G. Schendel, Z. Anorg. Chem. 1931, 196, 160.
- ↑ A. Weiss, D. R. Harvey, 1964, Angew. Chem. 76, 818.
- ↑ S. Sjöberg, N. Ingri, A. M. Nenner, L. O. Öhman, J. Inorg. Biochem. 1985, 24, 267
- ↑ Kinrade, S. D. et al. Science, 1999, 285, 1542-1545.
- ↑ Organosilicon Chemistry S. Pawlenko Walter de Gruyter New York 1986
- ↑ Lambert, J. B.; Lu, G.; Singer, S. R.; Kolb, V. M. J. Am. Chem. Soc. 2004, 126, 9611-9625.
- ↑ Handbook of Chemistry and Physics, 81st Edition CRC Press ISBN 0-8493-0481-4
- ↑ Mechanistic insight into copper-catalysed allylic substitutions with bis(triorganosilyl) zincs. Enantiospecific preparation of -chiral silanes Eric S. Schmidtmann and Martin Oestreich Chem. Commun., 2006, 3643 - 3645, Template:DOI
- ↑ By isotopic desymmetrisation on the substrate (replacing hydrogen by deuterium) it can be demonstated that the reaction proceeds not through the symmetrical π-allyl intermediate 5 which would give an equal mixture of 3a and 3b but through the Π-δ intermediate 4 resulting in 3a only, through an oxidative addition / reductive elimination step
- ↑ Radical Reduction of Aromatic Azides to Amines with Triethylsilane Luisa Benati, Giorgio Bencivenni, Rino Leardini, Matteo Minozzi, Daniele Nanni, Rosanna Scialpi, Piero Spagnolo, and Giuseppe Zanardi J. Org. Chem.; 2006; 71(15) pp 5822 - 5825; (Note) Template:DOI
- ↑ From Carbon Dioxide to Methane: Homogeneous Reduction of Carbon Dioxide with Hydrosilanes Catalyzed by Zirconium-Borane Complexes Tsukasa Matsuo and Hiroyuki Kawaguchi J. Am. Chem. Soc.; 2006; 128(38) pp 12362 - 12363; Template:DOI