Mustard gas: Difference between revisions
imported>David E. Volk mNo edit summary |
imported>David E. Volk |
||
| Line 15: | Line 15: | ||
== Chemistry of mustard gasses == | == Chemistry of mustard gasses == | ||
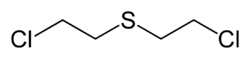
The chemistry and health effects of mustard gas have been thoroughly studied and comprehensive reviews are available.<ref name = "Veterans at Risk">{{cite book | title = Veterans at Risk: Health Effects of Mustard Gas and Lewisite | authors = Constance M Pechura and David P. Rallyear | publisher = Institute of Medicine | year = 1993}} </ref> | The chemistry and health effects of mustard gas have been thoroughly studied and comprehensive reviews are available.<ref name = "Veterans at Risk">{{cite book | title = Veterans at Risk: Health Effects of Mustard Gas and Lewisite | authors = Constance M Pechura and David P. Rallyear | publisher = Institute of Medicine | year = 1993}} </ref> Both mustard gas and the related amine gas weapons generally have a central [[Lewis base]] (electron donor), either an atom or a group, that can undergo [[nucleophilic attack]] of the terminal halogenated carbon(s) to form a cyclic cation ([[phosphonium]] [[ammonium]], or [[oxonium) with subsequent release of a halogen ion. The reactive phosphonium cation then reacts with water, opening the cyclic intermediate to form the hydroxide analog of the starting compound and releasing an acidic proton. Thus, the overall reaction releases [[hydrochloric acid]] (HCl). The reactive cyclic phosphonium ion also serves as an efficient alkylating reagent and can therefore react with, and attach to, biological chemicals such as [[DNA]] and [[protein]]s. Even if one subjected to mustard gas survives the initial blistering tissue damamge, subsequent cancers arising from DNA damage (alkylation, cross-linking) often lead to later death. | ||
| Line 24: | Line 24: | ||
:'''Levinstein Method''' SCl<sub>2</sub> + 2 H<sub>2</sub>C=CH<sub>2</sub> --> (ClCH<sub>2</sub>CH<sub>2</sub>)<sub>2</sub>S | :'''Levinstein Method''' SCl<sub>2</sub> + 2 H<sub>2</sub>C=CH<sub>2</sub> --> (ClCH<sub>2</sub>CH<sub>2</sub>)<sub>2</sub>S | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 11:35, 28 November 2010
Mustard gas refers to a family of potentially lethal but primarily casualty-producing chemical weapons introduced in the First World War (WW1) and subsequently improved in World War II (WWII). The first version was impure dichloroethyl sulfide, code-named "Yellow Cross" by the Germans and "H" in the modern U.S. system. Originally produced by the Lowenstein reaction, it could be purified by distillation into the more potent "HD".
During WWII, the U.S. developed a family of much more toxic nitrogen mustards gasses, called the "HN" series. After their declassification at the end of the war, they proved to be the basis of some of the first effective antineoplastic agents for cancer chemotherapy.
World War I
While it had been synthesized in 1860, mustard gas was first used in warfare in September 1917. German forces employed it against Russians at Riga. Considerably more toxic by weight than earlier chemical weapons, it became a regular part of bombardments until the end of the war.
As opposed to other agents in use at that time, it could penetrate unbroken skin. A mask alone was insufficient protection. Its onset of toxic effects were usually delayed, so there was a terrifying sense of uncertainty after being shelled.
World War II
Both sides stockpiled mustard but did not use it, with the possible exception of the Japanese against the China. There was, however, a major mustard gas contamination incident in Bari, Italy when German aircraft unknowingly bombed an ammunition ship carrying the theater reserve of the agent.
Germany conducted the Nazi mustard gas experiments on concentration camp prisoners.
Chemistry of mustard gasses
The chemistry and health effects of mustard gas have been thoroughly studied and comprehensive reviews are available.[1] Both mustard gas and the related amine gas weapons generally have a central Lewis base (electron donor), either an atom or a group, that can undergo nucleophilic attack of the terminal halogenated carbon(s) to form a cyclic cation (phosphonium ammonium, or [[oxonium) with subsequent release of a halogen ion. The reactive phosphonium cation then reacts with water, opening the cyclic intermediate to form the hydroxide analog of the starting compound and releasing an acidic proton. Thus, the overall reaction releases hydrochloric acid (HCl). The reactive cyclic phosphonium ion also serves as an efficient alkylating reagent and can therefore react with, and attach to, biological chemicals such as DNA and proteins. Even if one subjected to mustard gas survives the initial blistering tissue damamge, subsequent cancers arising from DNA damage (alkylation, cross-linking) often lead to later death.
Synthesis
The prototypical mustard gas, bis-(2-chloroethyl)sulfide, can be synthesized several different methods.The chemistry and health effects of mustard gas have been thoroughly studied and comprehensive reviews are available.[2]. Pure sulfur mustard can be prepared using Meyer's method of reacting thiodiglycol with phosphorus trichloride (PCl3) or other chlorinating agents including phosgene, thionyl chloride or concentrated hydrochloride. An alternative approach, which was used to produced wartime product, is to use the Levinstein method in which sulfur dichloride is reacted with ethylene to first produce 2-chloroethylsulfenyl chloride which is converted by a second addition of ethylene to mustard gas.
- Meyer Method 3 (HO-CH2=CH2)-S-(CH2=CH2-OH) + 2 PCl3 --> 3 S(CH2CH2Cl)2 + 2 P(OH)3
- Levinstein Method SCl2 + 2 H2C=CH2 --> (ClCH2CH2)2S