Cysteine
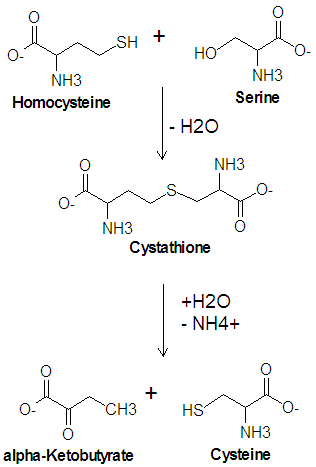
Cysteine is one of the twenty common amino acids. It is one of two amino acids which contain a sulfur atom, the other being methionine, and is one of the two amino acids which contain a hydroxyl group, the other being threonine. Cysteine is a precursor of methionine in the activated methyl cycle, and it is synthesized from a condensation reaction between the amino acid serine and homocysteine.
Biosynthesis
The enzyme cystathionine synthase catalyzes the condensation reaction of serine with homocysteine which produces cystathione and water. Another enzyme, cystathionase, then catalyzes the deamination and cleavage of cystathione to produce cysteine and -ketobutyrate. In this cleavage reaction, serine acts as the carbon skeleton and homocysteine provides the sulfur atom.