Click chemistry
Click chemistry, for which K. Barry Sharpless won the Nobel Prize in Chemistry, refers to a variety of synthetic chemistry methods in which complex molecules are synthesized by first synthesizing two complex pieces which are capable of clicking together in a reaction to form the often very complex final desired product. The term is most often associated with a reaction between an alkyne and an azide under mild conditions using a copper catalyst.
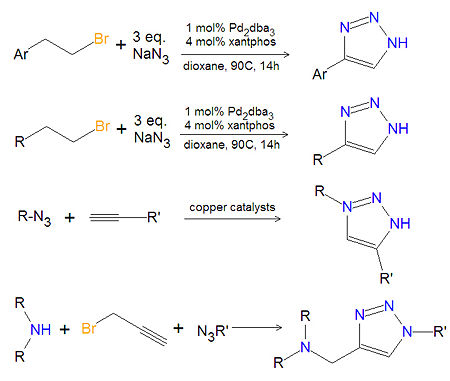
Synthesis of substituted 1,2,3-triazoles
Most substituted triazoles are synthesized by one of several click chemistry methods. One method uses palladium (Pd) catalysts to couple alkenyl halides and sodium azide to product 4-substituted-1,2,3-triazoles[1]. This reaction also works with aromatic alkenyl halides. Click chemistry between azides and terminal aklynes, catalyzed by various copper reagents, can be used to synthesize 3,5-disubstituted-1,2,3-triazoles[2],[3].
A variety of 1,4-disubstituted-1,2,3-triazoles can be made economically and in an environmentally friendly manner, by reacting a secondary amine, propargyl halides and an azide together in water, under atmospheric oxygen with a copper catalyst.[4]. Another method of 1,4-disubstituted-1,2,3-triazoles (not shown) uses no solvent. In this method, the cycloaddition of alkyl azides with enol ethers provides triazoles that are not amendable to synthesis using the coupling of azides with alkynes.[5] A variety of other synthetic methods are available[6].
References
- ↑ Barluenga J et al. (2006). "Developments in Pd catalysis: synthesis of 1H-1,2,3-triazoles from sodium azide and alkenyl bromides". Angew Chem Int Ed 45: 6893-6. DOI:10.1002/anie.200601045. Research Blogging.
- ↑ Rostovtsev VV et al. (2002). "Huisgen 1,3-Dipolar Cycloaddition". Angew Chem 114: 2708-11. DOI:<2708::AID-ANGE2708>3.0.CO;2-0 10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0. <2708::AID-ANGE2708>3.0.CO;2-0 Research Blogging.
- ↑ Lipshutz BH, Taft BR (2006). "Heterogeneous copper-in-charcoal-catalyzed click chemistry". Angew Chem Int Ed 45: 8235-8. DOI:10.1002/anie.200603726. Research Blogging.
- ↑ Yan et al. (2005). "Efficient synthesis of 1,4-disubstituted 1,2,3-triazoles in ionic liquid/water system". Tetrahedron 61: 9331-7. DOI:10.1016/j.tetlet.2006.01.004. Research Blogging.
- ↑ Rogue DR et al. (2005). "Synthesis of 1,2,3-triazoles by cycloadditions of azides with enol ethers". Synthesis 2005: 2497-2502. DOI:10.1055/s-2005-872116. Research Blogging.
- ↑ 1,2,3-triazole synthesis. Retrieved on 2008-05-17.