Cephalosporin: Difference between revisions
imported>David E. Volk No edit summary |
imported>Howard C. Berkowitz (Fourth generation started) |
||
| (9 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
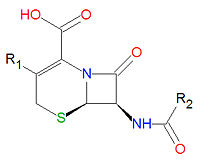
{{Image|Cephalosporin base.jpg|right|200px|Base structure of all cephalosporins.}} | |||
''' | '''Cephalosporins''' are a class of [[antibiotic]] compounds sharing a common base structure, 7-aminocephalosporanic acid (7-ACA), that was derived from the first cephalosporin discovered, [[cephalosporin C]]. [[Penicillin]]s are very similar, although they contain a five-membered ring in place of the six-membered ring present in the cephalosporin. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of a [[lactam|beta-lactam]], which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins are often made semisynthetically. Cephalosporins and the very closely related[[cephamycin]]s are collectively referred to as [[cephems]]. | ||
== Nomenclature == | |||
Because the original cephalosporins used the "ceph" form of the spelling and were often trademarked, the [[International Nonproprietary Name]]s (INN) suggested by the [[World Health Organization]] use the "cef" spelling for the generic drug name of all cephalosporins. | |||
== First generation cephalosporins == | == First generation cephalosporins == | ||
| Line 36: | Line 39: | ||
== Third generation cephalosporins == | == Third generation cephalosporins == | ||
The third generation has additional activity against ''[[Pseudomonas aeruginosa]]''. | |||
* [[Cefcapene]] | * [[Cefcapene]] | ||
* [[Cefdaloxime]] | * [[Cefdaloxime]] | ||
| Line 44: | Line 48: | ||
* [[Cefmenoxime]] | * [[Cefmenoxime]] | ||
* [[Cefodizime]] | * [[Cefodizime]] | ||
* [[Cefoperazone]] | |||
* [[Cefotaxime]] | * [[Cefotaxime]] | ||
* [[Cefpimizole]] | * [[Cefpimizole]] | ||
* [[Cefpodoxime]] | * [[Cefpodoxime]] | ||
* [[Ceftazidime]] | |||
* [[Cefteram]] | * [[Cefteram]] | ||
* [[Ceftibuten]] | * [[Ceftibuten]] | ||
| Line 53: | Line 59: | ||
* [[Ceftizoxime]] | * [[Ceftizoxime]] | ||
* [[Ceftriaxone]] | * [[Ceftriaxone]] | ||
== Fourth generation cephalosporins== | |||
* [[ | While still active against ''[[Enterobacteriaceae]]'' and ''[[Pseudomonas aeruginosa]]'', this class has much better activity against gram-positive organisms than the third-generation antipseudomonals. The single approved agent is less effective against [[methicillin-resistant Staphylococcus aureus]] than the first and second generations. It is not effective against ''[[Bacteroides fragilis]]''. | ||
* [[Cefepime]] | |||
Latest revision as of 21:37, 2 May 2010
Cephalosporins are a class of antibiotic compounds sharing a common base structure, 7-aminocephalosporanic acid (7-ACA), that was derived from the first cephalosporin discovered, cephalosporin C. Penicillins are very similar, although they contain a five-membered ring in place of the six-membered ring present in the cephalosporin. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of a beta-lactam, which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins are often made semisynthetically. Cephalosporins and the very closely relatedcephamycins are collectively referred to as cephems.
Nomenclature
Because the original cephalosporins used the "ceph" form of the spelling and were often trademarked, the International Nonproprietary Names (INN) suggested by the World Health Organization use the "cef" spelling for the generic drug name of all cephalosporins.
First generation cephalosporins
- Cefacetrile
- Cefadroxil
- Cefalexin
- Cefaloglycin
- Cefalonium
- Cefaloridine
- Cefalotin
- Cefapirin
- Cefatrizine
- Cefazaflur
- Cefazedone
- Cefazolin
- Cefradine
- Cefroxadine
- Ceftezole
Second generation cephalosporins
In general, second generation cephalosporins have a broader spectrum of activity against Gram-negative bacteria.
Third generation cephalosporins
The third generation has additional activity against Pseudomonas aeruginosa.
- Cefcapene
- Cefdaloxime
- Cefdinir
- Cefditoren
- Cefetamet
- Cefixime
- Cefmenoxime
- Cefodizime
- Cefoperazone
- Cefotaxime
- Cefpimizole
- Cefpodoxime
- Ceftazidime
- Cefteram
- Ceftibuten
- Ceftiofur
- Ceftiolene
- Ceftizoxime
- Ceftriaxone
Fourth generation cephalosporins
While still active against Enterobacteriaceae and Pseudomonas aeruginosa, this class has much better activity against gram-positive organisms than the third-generation antipseudomonals. The single approved agent is less effective against methicillin-resistant Staphylococcus aureus than the first and second generations. It is not effective against Bacteroides fragilis.