Benzene: Difference between revisions
Jump to navigation
Jump to search

imported>Paul Derry (New image, and a rewrite of the summary. More later.) |
imported>Howard C. Berkowitz No edit summary |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
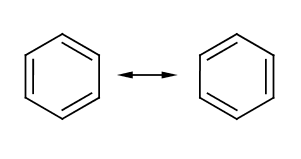
[[Image:Chemistry - Benzene - Kekule Structure.png|right|thumb|350px|{{#ifexist:Template:Chemistry - Benzene - Kekule Structure.png/credit|{{Chemistry - Benzene - Kekule Structure.png/credit}}<br/>|}}The commonly recognized, but somewhat inaccurate Kekulé representation of Benzene | [[Image:Chemistry - Benzene - Kekule Structure.png|right|thumb|350px|{{#ifexist:Template:Chemistry - Benzene - Kekule Structure.png/credit|{{Chemistry - Benzene - Kekule Structure.png/credit}}<br/>|}}The commonly recognized, but somewhat inaccurate Kekulé representation of Benzene, which Kekulé said came to him in a dream]] | ||
'''Benzene''' (C<sub>6</sub>H<sub>6</sub>) is a six carbon aromatic compound commonly used in industry as a precursor for other important aromatics such as toluene, or benzoic acid. The structure of benzene could not easily be determined due to its unusual electronic characteristics. | '''Benzene''' (C<sub>6</sub>H<sub>6</sub>) is a six carbon aromatic compound commonly used in industry as a precursor for other important aromatics such as toluene, or benzoic acid. The structure of benzene could not easily be determined due to its unusual electronic characteristics. | ||
Revision as of 09:28, 4 September 2008
Benzene (C6H6) is a six carbon aromatic compound commonly used in industry as a precursor for other important aromatics such as toluene, or benzoic acid. The structure of benzene could not easily be determined due to its unusual electronic characteristics.