Azole: Difference between revisions
imported>David E. Volk (New page: {{subpages}} right|thumb|250px|{{#ifexist:Template:Azole structures.jpg/credit|{{Azole structures.jpg/credit}}<br/>|}}Structures of common azole compounds. ...) |
imported>David E. Volk mNo edit summary |
||
| Line 2: | Line 2: | ||
[[Image:Azole structures.jpg|right|thumb|250px|{{#ifexist:Template:Azole structures.jpg/credit|{{Azole structures.jpg/credit}}<br/>|}}Structures of common azole compounds.]] | [[Image:Azole structures.jpg|right|thumb|250px|{{#ifexist:Template:Azole structures.jpg/credit|{{Azole structures.jpg/credit}}<br/>|}}Structures of common azole compounds.]] | ||
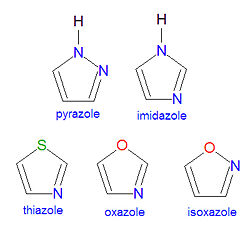
An '''azole''' is a chemical compound containing a five-membered [[aromatic]] ring structure with two heteroatoms, at least one of which must be a nitrogen atom. One, and only one, lone pair of electrons from each heteroatom is part of the aromatic bonding in an azole. Examples of common azole compounds include [[pyrazole]], [[imidazole]], [[thiazole]], [[oxozole]] and [[isoxazole]], shown in the illustration. The name or suffix azole changes upon [[saturation]] of the double bonds. When one of the double bonds becomes saturated, the resulting compound name changes its ending from ''azole'' to ''azoline'', and further reduction of the second double bond produces an ''azolidine''. Thus, the saturation of one double bond of oxazole yields 2-[[oxazoline]] and a further reduction yields [[oxazolidine]]. Aromatic heterocyclic systems with two nitrogens exist, and two of them, [[pyrimidine]] and [[purine]], are particularly important biochemicals. | An '''azole''' is a chemical compound containing a five-membered [[aromatic]] ring structure with two heteroatoms, at least one of which must be a nitrogen atom. One, and only one, lone pair of electrons from each heteroatom is part of the aromatic bonding in an azole. Examples of common azole compounds include [[pyrazole]], [[imidazole]], [[thiazole]], [[oxozole]] and [[isoxazole]], shown in the illustration. The name or suffix azole changes upon [[saturation]] of the double bonds. When one of the double bonds becomes saturated, the resulting compound name changes its ending from ''azole'' to ''azoline'', and further reduction of the second double bond produces an ''azolidine''. Thus, the saturation of one double bond of oxazole yields 2-[[oxazoline]] and a further reduction yields [[oxazolidine]]. Aromatic heterocyclic systems with two nitrogens exist, and two of them, [[pyrimidine]] and [[purine]], are particularly important biochemicals. The number of ring atoms in azoles starts with the heteroatom that is not part of a double bond, and then proceeds towards the other heteroatom. | ||
Revision as of 17:08, 8 February 2008
An azole is a chemical compound containing a five-membered aromatic ring structure with two heteroatoms, at least one of which must be a nitrogen atom. One, and only one, lone pair of electrons from each heteroatom is part of the aromatic bonding in an azole. Examples of common azole compounds include pyrazole, imidazole, thiazole, oxozole and isoxazole, shown in the illustration. The name or suffix azole changes upon saturation of the double bonds. When one of the double bonds becomes saturated, the resulting compound name changes its ending from azole to azoline, and further reduction of the second double bond produces an azolidine. Thus, the saturation of one double bond of oxazole yields 2-oxazoline and a further reduction yields oxazolidine. Aromatic heterocyclic systems with two nitrogens exist, and two of them, pyrimidine and purine, are particularly important biochemicals. The number of ring atoms in azoles starts with the heteroatom that is not part of a double bond, and then proceeds towards the other heteroatom.