Aluminium trichloride: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk mNo edit summary |
imported>David E. Volk No edit summary |
||
| Line 3: | Line 3: | ||
{{Chem infobox | {{Chem infobox | ||
|align=right | |align=right | ||
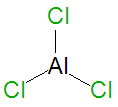
|image= | |image= {{Image|AlCl3.png|right|350px|AlCl<sub>3</sub>, a [[Lewis acid]].}} | ||
|width=350px | |width=350px | ||
|molname=Aluminum trichlride | |molname=Aluminum trichlride | ||
Revision as of 10:29, 4 July 2009
|
| |||||||
| Aluminum trichlride | |||||||
| |||||||
| Uses: | catalyst, astringent | ||||||
| Properties: | pyrophoric Lewis acid | ||||||
| Hazards: | spontaneously ignites in air | ||||||
| |||||||
Aluminum trichloride, AlCl3, is an important industrial chemical catalyst, especially for polymerization reactions. It is a pyrophoric Lewis acid chemical compound which instantly ignites when it is exposed to air. It is a highly reactive compound that can also be used as a chemical promoter in chelation-controlled reactions. It is also an astringent, used medically for athlete's foot hyperhidrosis therapy.[1]