Enantiomer
In chemistry, enantiomers, originally called optical isomers, are chemical structures that are nonsuperimposible mirror images of each other. In biochemistry, many chemicals are useful in only one of the two (or more) possible isomeric forms. For example, the levorotary (L or +) isomers of the amino acids are used to build proteins, but not the dextrorotary (R or -) isomers. Not all isomers are optically active (see #geometric isomers). This concept is easily understood by looking at one's own hands. The human left and right hands are nonsuperimposible mirror images of each other. There is no way to rotate the left hand to be exactly like the right hand, but the mirror image of the left hand looks exactly like the right hand.
Simplest example
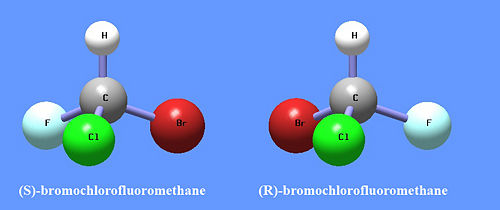
The simplest example of an enantiomer is an atom bound to four different substituents. Consider the isomeric structures of (R)- and (S)-bromochlorofluoromethane, shown here. These two chemicals have exactly the same atomic substituents, but they are arranged slightly differently, and they are mirror images of each other. The structure on the left cannot be made to look like the one on the right by any rotations. These molecules have only one stereocenter, but more complex molecules can have multiple stereocenters and therefore they can form many different diasteriomers.
Absolute configuration
Before the absolute structures of compounds could be determined, isomers were named by the rotation of polarized light either to the left (levorotary, or L) or to the right (dextrorotary, or R) that isomers caused. These names are now denoted as (-) for levorotary and (+) for dextrorotary, to avoid confusion with the names which gives absolute configuration information, as the above example does. When the actual structure of a chemical isomer is known, it is named according to the Cahn-Ingold-Prelog rules. It is important to note that the absolute configuration and the optical activity designation are not related. If the optical activity and polarization are known, then both designations are included in the names, such as (S)-(+)-alanine and (R)-(–)-alanine.