User talk:Paul Wormer/scratchbook
A barometric formula gives the pressure in a planetary atmosphere—usually the Earth's atmosphere—as a function of height. Since the temperature and composition of the atmosphere may be a complicated function of height, a barometric formula may be very intricate.
However, two simplified formulas are in common use. The first one assumes constant temperature and composition throughout the atmosphere. It assumes that the atmosphere is an ideal gas. The equation is a form of a Boltzmann distribution,
Here p(z) is the pressure at height z; z0/sub> is a suitable reference height, often the surface of the planet; m is the mass of one mole of the atmospheric gas (note that the equation holds for one substance only, if the atmosphere contains more than one compound a weighted mass is used); g is the gravitational acceleration, R is the molar gas constant; T is the absolute temperature (assumed independent of z).
The second barometric formula is based on the same assumptions.
Proof
Differential equation
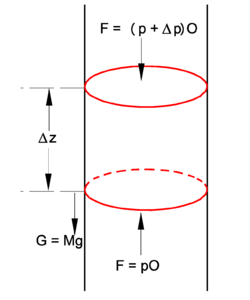
In the figure we see a stationary pillbox at arbitrary position z in a cylinder filled with air. The pillbox is pushed up by a pressure force pO, and pulled down by gravitation, which gives a force G equal to the total mass M of air in the pillbox times the gravitational acceleration g. Also the air pressure (p+ Δp)O at the top acts downward. Forces upward are positive, downward negative, and since the total force is zero (no motion of air), we have
The total mass in the pillbox is the molar density ρ times the molar mass m times the volume of the pillbox Δz·O
Hence
Noting that the molar density is the number of moles per volume, we get for an ideal gas,
Assuming that the gas in the pillbox is ideal and of absolute temperature T, we get the equation
Taking the limit of infinitesimal small Δ and remembering that the derivative of the natural logarithm is given by
we arrive at the the differential equation
Constant temperature
The master equation (1) can be solved by integrating the left hand side from p0 to p and the right hand side from z0 to z. Hence
where we recall that
Taking the exponent (the inverse function of the natural logarithm) of both sides of equation (2) we get
This distribution is known in statistical thermodynamics as a Boltzmann distribution.
Temperature linear in height
We assumed in the previous subsection that T is constant throughout the cylinder, which makes the equation useful for cases where there is no temperature gradient, i.e., case where the temperature at the top of the cylinder is equal to the temperature at the bottom. The master equation (1) can be modified for the case of a constant temperature gradient (constant lapse rate). That is, we make the substitution
in equation (1). The modified equation is still easily integrated:
Substitute
The integral on the right hand side becomes
We already saw that the integral on the left hand side is the logarithm of p/p0. hence
![{\displaystyle p(z)=p(z_{0})\;\exp[-{\frac {mg}{RT}}(z-z_{0})]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b465627ecb389375864ff7d689b9fbf44df53d8d)









![{\displaystyle {\frac {p}{p_{0}}}={\frac {\exp \left[-{\frac {mg}{RT}}z\right]}{\exp \left[-{\frac {mg}{RT}}z_{0}\right]}}\;\Longleftrightarrow \;p=p_{0}\,\exp \left[-{\frac {mg}{RT}}(z-z_{0})\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/218b3157c56c311be32e3c191ebe8f04e95bca1b)



![{\displaystyle -{\frac {mg}{R}}\int _{z_{0}}^{z}{\frac {1}{T+K(z'-z_{0})}}dz'=-{\frac {mg}{KR}}\int _{T}^{T+K(z-z_{0})}{\frac {dy}{y}}=-{\frac {mg}{KR}}\ln {\frac {T+K(z-z_{0})}{T}}=\ln \;\left[{\frac {T}{T+K(z-z_{0})}}\right]^{\frac {mg}{KR}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e638c09a6ccecf62589bc5089943d3f35f973738)
![{\displaystyle {\frac {p}{p_{0}}}=\left[{\frac {T}{T+K(z-z_{0})}}\right]^{\frac {mg}{KR}}.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/99589c9e1f2501522593a25bed93957071412a71)