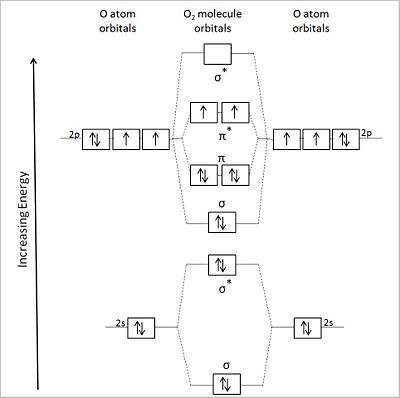
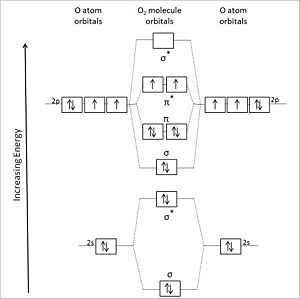
User:Anthony.Sebastian/SebastianSandbox-2
Osteoporosis
Surgeon General (Smith 2000) [1]
Mole (Chemistry)
Chemists employ standard units of measurement to characterize samples of the substances they study. For example:
- they use the basic unit designated liter (abbr., L) to characterize the volume of the sample, viz., the amount of three-dimensional space the sample occupies. Under the same conditions, 1.45 L of water occupies the same volume as 1.45 L of ethanol (drinking alcohol).
- they use the basic unit designated kilogram (abbr., kg) to characterize the mass of the sample, viz., the quantity of matter in the sample as reflecting the force required to accelerate it (force equals mass times acceleration, F=ma). 1.52 kg of water contains the same mass of matter as 1.52 kg of ethanol.
Often chemists want to characterize a sample of a substance by a unit of measurement that represents the number of molecules the sample contains. Here we will discuss that unit in relation to pure substances, e.g., those that contain only one type of molecule, as in the case of samples of pure water, pure glucose, pure dioxygen, etc. — namely, chemical compounds.
For the purpose, chemists invented the basic unit designated mole. Just as equal liters of different chemical compounds occupy the same volume, and equal masses of different chemical compounds contain the same quantity of matter, equal moles of different chemical compounds contain the same number of molecules.
Without a deep understanding of the concept and application of the mole, one cannot exploit chemistry to develop understanding biology and medicine, or to produce industrial and technological advances.
In this article, we will describe how chemists define the unit, mole, in a way that allows it to reflect the number of molecules in a sample of a chemical compound; how they determine the number of moles in a sample; how they use the unit to characterize chemical reactions and for many other purposes; and how they extend use of the unit to samples of chemical elements and of other types of pure chemical substances besides compounds and elements.
testing refs
abc[2]
def[3]
Creeping up on moles
The mass of single atoms of the chemical elements
A molecule of a chemical compound consists of two or more different types of atoms (e.g., hydrogen and oxygen atoms when considering a water molecule) bound together in a fixed ratio per molecule (e.g., two hydrogen atoms to one oxygen atom per molecule of water). To understand how the unit of measurement, mole, gives a representation of the number of molecules in a sample of a chemical compound (e.g., 1.0 L of water; 1.0 kg of dioxygen gas), we first need to know the masses of the atoms that make up the sample.[4] Each of the 92 different types of atoms (making up the 92 naturally-occurring elements) has a different 'mass number', referred to as the atomic mass number. Moreover, the atoms of many elements exist as multiple 'isotopes' that have differing number of neutrons, hence in atomic mass number, accompanying their element-unique number of protons and electrons.
Using atomic mass numbers to calculate the number of molecules in samples of chemical compounds
If we knew the mass numbers of the different types of atoms in a chemical compound, [5] the relative abundance and mass numbers of the several isotopes of those element’s atoms, the ratio of the different types of atoms making up the molecules of the chemical compound, and the total mass of the sample of the chemical compound, we could, with simple algebra, calculate the number of molecules in the sample.
Consider 1.0 g (1/1000th of a kg) of water, each molecule of which consists of two hydrogen atoms and one oxygen atom. Almost all hydrogen atoms exist as the isotope that has one proton and one electron (no extra neutrons), each atom with a mass of 1.674 x 10-21 kg, or 1.674 x 10-24 g, where one g equals 1/1000th of a kg (=10-3 kg). Almost all oxygen atoms exist as the isotope that has eight protons, eight neutrons, and eight electrons, designated O-16, each atom with a mass of 2.655 x 10-23 g. Ignoring the minor isotopes, and considering that water consists of two hydrogen atoms and one oxygen atom per molecule, we calculate the mass of a molecule of water as 2 x 1.674 x 10-24 + 2.655 x 10-23, or 2.990 x 10-23 g/molecule. 1.0 g of water then must contain (1.0 g /2.990 x 10-23 g/molecule), or 3.34 x 1022 molecules.
Scaling up atomic mass numbers for convenience: the 'atomic mass unit'
From the previous section we can conclude that a fifth of a teaspoon of water (~1.0 g) contains about 300 billion times more molecules than the number of stars in our galaxy, a consequence of the minute masses of its atoms. In fact, for all elements, atomic mass numbers range from about 10<sup-24 to 10<sup-22 g, all very minute. For convenience, chemists minimize dealing with such small numbers by defining a unit that serves as a marker, or conversion factor, for the atomic mass numbers. They call it the “atomic mass unit” (abbr., amu), and define it as 1/12 of the mass of one atom of the most abundant isotope of carbon, carbon-12 (6 protons, 6 neutrons, six electrons), the latter equal to 1.9926 x 10-23 g, 1/12th of which equals 1.6605 x 10-24 g. Thus,
Using that formula as a conversion factor, one can compute how many atomic mass units (amu’s) for any atom, knowing the atom’s mass number. O-16, with a mass number as mentioned of 2.655 x 10-23 g, must have [(2.655 x 10-23 g/atom)/1.6605-24 g/amu)] amu, or 15.99 amu. Thus, an oxygen atom has a mass of 15.99, expressed as atomic mass units. Knowing the conversion formula, we can always get back to oxygen’s mass in grams.
Converting atomic mass numbers to atomic mass units still allows us to determine the number of molecules in a sample of a chemical compound. We use the same procedure as we employed using atomic mass numbers, except now we use the more convenient small numbers of atomic mass units.
The minor isotopes: a little more chemistry before we can grasp the mole
Ignoring the minor isotopes of the atoms in the molecule of a chemical substance, as we did in the earlier example of calculating the number of molecules in a sample of a chemical compund, will not allow us to accurately calculate the number of molecules in the sample. Without incorporating them, we will not know the average atomic mass of a given atom type that has isotopes with slightly differing atomic mass units. To incorporate all of an element’s isotopes in getting the element’s atoms’ average atomic mass we must know the atomic mass units of each isotope, as well as its relative abundance among all the isotopes of the element. With a little algebra, we can then calculate the average mass of the atoms of a given element, and express the results in atomic mass units, for convenience.
Logically we would call the result the atomic mass of the element’s atoms, averaged out for all of the element’s isotopes. However, by tradition chemists call the result the atomic weight (AW) of the element’s atoms, the number we find in the periodic table.
For a simple example of how to calculate the atomic weight of an element, consider the element, oxygen. Oxygen has three naturally occurring isotopes of its atoms: O-16, amu=15.99491463, abundance=99.757%; O-17, amu=16.9991312, abundance=0.038%; O-18, amu=17.9991603, abundance=0.205%. We can calculate how much each isotope contributes the mass of the element’s atoms by the product of its fractional abundance and its atomic mass expressed in atomic mass units. By adding together those products we then get atomic mass of the element’s atoms averaged among the several isotopes, as if all the atoms had the same atomic mass. For oxygen, the calculation can be written as:
We see that number as the atomic weight of oxygen in the periodic table.
Grasping the mole
We now have nearly all we need to grasp the concept of mole. We just need a formal definition of mole. In defining the mole, chemists have done something similar to defining atomic mass units: they devised a definition that allow expressing large numbers (e.g., 1020) of molecules in convenient small numbers.
Without background of history or rationale at this point, we give the formal definition of mole:
One mole (of any chemical compound, say) equals the number of atoms in exactly 12 g of the major isotope of carbon, carbon-12.
- That definition tells us immediately that one mole of every chemical compound has the same number of molecules as the number of atoms in one mole, or 12 g, of carbon-12.
Just how many atoms does one mole, or 12 g, of cabon-12 have? We showed in an earlier section how to calculate the number of molecules in a given sample of a chemical compound. We can use the same method for determining how many atoms in a given sample, 12 g, of carbon-12.
- First we obtain from an appropriate look-up table the mass (i.e., atomic mass number) of one atom of carbon-12. We obtain 1.9926 x 10-23 g/ carbon-12 atom.
- Our sample contains 12 g carbon-12 atoms.
- For 12 g carbon-12, and 1.9926*10<su>-23 g per carbon-12 atom, with grams cancelling out, we can write:
- 12 g carbon-12/1.9226*10-23 g/carbon-12 atom = 6.022*1023 carbon-12 atoms
Thus, 12 g carbon-12 contains 6.022*1023 atoms.
Since the number of atoms in 12 g carbon-12 by definition equals one mole of carbon-12, and one mole of any chemical compound has the same number of molecules as does one mole of carbon-12, one mole of any chemical compound contains 6.022*1023 molecules.
Although chemists define the mole in terms of carbon atoms, the mole unit (6.022*1023 carbon atoms) applies to any sample of a pure substance (chemical compounds, atoms, ion, electrons, quanta, etc.) — each of which contains 6.022*1023 of its constituents, just as one score means 20 of any item (see Gettysburg Address) and one dozen means 12 of any item. In the chemistry of pure substances one mole means 6.022*1023 of any constituent.
If we remember that we can interconvert atomic mass numbers, ranging from 10-22 to 10-24 g, to atomic mass units (amu’s), ranging more conveniently from ~1 to ~240 amu, we can also use amu’s to calculate the number of items in one mole.
- One amu = 1/12th of the atomic mass number of one carbon-12 atom, the latter equal to 1.9926*10-23, 1/12th of which equals 1.6605*10-24 g (viz., .9926*10-23 g/12).
- Since one amu is 1/12th the atomic mass number of one carbon-12 atom, that means one carbon-12 atom has a mass in amu’s 12 times that, or precisely 12 amu.
- The number of atoms in a sample of 12 g carbon-12 then computes as:
Again, then 12 g carbon-12, or one mole of carbon-12, has 6.022*1023 atoms.
Chemists call the number of constituent items in one mole of an element or chemical compound Avogadro’s number
To repeat, 1 mole of atoms of every element equals 6.022*1023 atoms, and one mole of a sample of any pure substance (molecules, ions, electrons, radicals, quanta, etc.) likewise contains 6.022*1023 of its constituent items.
Chemists refer the number 6.022*1023 as Avogadro’s Number (NA), after the pioneering physicist, Amedeo Avogadro (1776-1856), who first recognized the distinction between atoms and molecules. To get a sense of the size of Avogadro’s number, the Oxford chemist, Peter Atkins called attention to the visualization that with an Avogadro’s number, one mole, of soft-drink cans one could cover the surface of the earth with a pile 200 miles high.
test

xxx[6]
refs
- ↑ Quite a guy!
- ↑ Alter SG. (2007) Darwin and the linguists: the coevolution of mind and language, Part 1. Problematic friends. Stud. Hist Philos. Biol. Biomed. Sci. 38:573-84. PMID 17893066.
- Abstract: : In his book The descent of man (1871), Charles Darwin paid tribute to a trio of writers (Hensleigh Wedgwood, F. W. Farrar, and August Schleicher) who offered naturalistic explanations of the origin of language. Darwin's concurrence with these figures was limited, however, because each of them denied some aspect of his thesis that the evolution of language had been coeval with and essential to the emergence of humanity's characteristic mental traits. Darwin first sketched out this thesis in his theoretical notebooks of the 1830s and then clarified his position in Descent, where he argued that mind-language coevolution had occurred prior to the rise of distinct racial groups. He thus opposed the view of August Schleicher and Ernst Haeckel, who (along with Alfred Russel Wallace) taught that speech had originated subsequent to the geographical and racial dispersion of humanity's ancestors. As Darwin argued in Descent, this quasi-polygenetic version of coevolution was unable to explain primeval man's initial dominance over rival ape-like populations. Drawing inspiration from British anthropologists, Darwin made the early development of language, hence mental monogenesis, central to his account of human evolution.
- ↑ Alter SG. (2007) Race, language, and mental evolution in Darwin's descent of man. J. Hist Behav. Sci. 43:239-55. PMID 17623873.
- Abstract: Charles Darwin was notoriously ambiguous in his remarks about the relationship between human evolution and biological race. He stressed the original unity of the races, yet he also helped to popularize the notion of a racial hierarchy filling the gaps between the highest anthropoids and civilized Europeans. A focus on Darwin's explanation of how humans initially evolved, however, shows that he mainly stressed not hierarchy but a version of humanity's original mental unity. In his book The Descent of Man, Darwin emphasized a substantial degree of mental development (including the incipient use of language) in the early, monogenetic phase of human evolution. This development, he argued, necessarily came before primeval man's numerical increase, geographic dispersion, and racial diversification, because only thus could one explain how that group was able to spread at the expense of rival ape-like populations. This scenario stood opposed to a new evolutionary polygenism formulated in the wake of Darwin's Origin of Species by his ostensible supporters Alfred Russel Wallace and Ernst Haeckel. Darwin judged this outlook inadequate to the task of explaining humanity's emergence.
- ↑ Note: This discussion requires accepting the standard units of mass, including atomic mass, as established by international agreement.
- ↑ Note: Chemists have devised methods for determining the masses of atoms (e.g., mass spectroscopy) and have compiled lists of the mass numbers of the various isotopes of the naturally-occurring elements.
- ↑ Ilberg J. (1927) Sorani Gynaeciorum Libri N. De signis fracturarum. De fascUs. Vita Hippocratis secundum Soranum (CMG IV), Leipzig & Berlin.
Image test
Risks of Developing Osteoporosis in Women and Men
Fractures, a common consequence of osteoporosis, and often the first indication of the disease, rank as osteoporosis' most adverse consequence. It of causes severe pain and debilitation, especially in the elderly who fall and fracture their hip, and it can lead to death from complications during the planned recovery period. Some 20% of hip fracture patients die within a year (Leibson et al. 2002).
The 1.5 million osteoporotic fractures in the United States each year lead to more than half a million hospitalizations, over 800,000 emergency room encounters, more than 2,600,000 physician office visits, and the placement of nearly 180,000 individuals into nursing homes. Hip fractures are by far the most devastating type of fracture, accounting for about 300,000 hospitalizations each year (Surgeon General 2004).
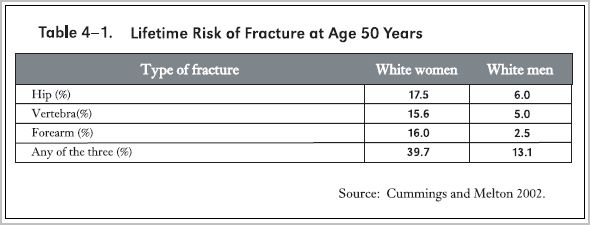
The accompanying table from the Surgeon General's 2004 report (Surgeon General 2004) indicates that at age 50 years white women carry a lifetime risk of hip, spine or forearm fracture amounting to nearly 40%, and men about 13%.
The gender difference relates in part to the faster waning of sex steroid hormones in women as they age, menopause predating the more gradual andropause. Male and female sex hormones act on bone in a positive way, not surprisingly since successful reproduction depends in many ways on healthy bones in the parents.
References Cited
- Listed here in alphabetical order by last name of first author as cited with publication date in the text.
- Leibson CL, Tosteson AN, Gabriel SE, Ransom JE, Melton LJ. (2002) Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr. Soc. 50(10):1644-50. PMID 12366617.
- Abstract: OBJECTIVES: To compare persons with and without hip fracture for subsequent mortality and change in disability and nursing home (NH) use. DESIGN: Population-based historical cohort study. SETTING: Olmsted County, Minnesota. PARTICIPANTS: All residents who experienced a first hip fracture between January 1, 1989, and December 31, 1993, and, for each case, a resident of the same sex and similar age who had not experienced a hip fracture and was seen by a local care provider. MEASUREMENTS: Data on disability (Rankin score), comorbidity (Charlson Index), and NH residency before baseline (fracture date for cases and registration date for controls) were obtained by review of complete community-based medical records. The records were then reviewed from baseline through December 31, 1994, for Rankin disability at 1 month and 1 year, all NH admissions and discharges, and date of death for those who died. RESULTS: There were 312 cases and 312 controls (81% female, mean age +/- standard deviation = 81 +/- 12 years). Before baseline, cases had higher comorbidity (45% vs 30% had Charlson Index >/= 1, P <.001) and disability (mean Rankin score = 2.5 +/- 1.1 vs 2.2 +/- 1.1, P <.001) and were more likely to be in a NH (28% vs 18%, P <.001) than controls. One year after baseline, estimated mortality was 20% (95% confidence interval (CI) = 16-24) for cases vs 11% (95% CI = 8-15) for controls, 51% of cases versus 16% of controls had a level of disability one or more units worse than before baseline (P <.001), and the cumulative incidence of first NH admission was 64% (95% CI = 58-71) for cases versus 7% (95% CI = 4-11) for controls. The risk of NH admission for cases relative to controls diminished over time, but remained elevated 5 years after the event (risk ratio = 20.0 at 3 months and 2.1 at 5 years), but, in persons admitted to a nursing home, cases were two times more likely than controls to be discharged alive within a year (P <.001). CONCLUSIONS: Hip fracture is an important contributor to disability and NH use, but the potential savings from hip fracture prophylaxis may be overestimated by studies that fail to consider differential risk, mortality, and long-term follow-up.
- Cummings SR, Melton LJ. (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359(9319):1761-7 PMID 12049882.
- Abstract: Bone mass declines and the risk of fractures increases as people age, especially as women pass through the menopause. Hip fractures, the most serious outcome of osteoporosis, are becoming more frequent than before because the world's population is ageing and because the frequency of hip fractures is increasing by 1-3% per year in most areas of the world. Rates of hip fracture vary more widely from region to region than does the prevalence of vertebral fractures. Low bone density and previous fractures are risk factors for almost all types of fracture, but each type of fracture also has its own unique risk factors. Prevention of fractures with drugs could potentially be as expensive as medical treatment of fractures. Therefore, epidemiological research should be done and used to identify individuals at high-risk of disabling fractures, thereby allowing careful allocation of expensive treatments to individuals most in need.
- Surgeon General Report. (2004) Bone Health and Osteoporosis
Notes (numbered as footnotes in text)
Scientists
For biographies of scientists.
Table from OO
|
TIMETABLE OF EVENTS PERTINENT TO THE LIFE, WORK AND TIMES OF J. B. S. HALDANE (referred in this chronology as J.B.S.)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||