User:Milton Beychok/Sandbox
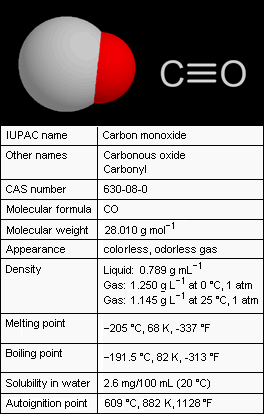
Carbon monoxide (CO), also referred to as carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. Exposure to high levels of carbon monoxide is extremely toxic to humans and animals. Conversely, small amounts of carbon monoxide are produced in normal animal metabolism and it is thought to have some normal biological functions.
Carbon monoxide consists of one carbon atom and one oxygen atom. It is the simplest member of the class of inorganic compounds known as oxocarbons which includes carbon dioxide (CO2), carbon suboxide (C3O2), mellitic anhydride (C12O9) and many others. When combined with a metal (i.e., an organometallic complex), the carbon monoxide is a ligand called carbonyl : for example, in nickel carbonyl with the formula Ni(CO)4.
Carbon monoxide is produced by the partial combustion of carbon-containing substances. It is produced when there is not enough oxygen to form carbon dioxide, such as when operating a stove or an internal combustion engine in an enclosed space.
In the presence of oxygen, carbon monoxide burns with a blue flame, producing carbon dioxide.[1] Coal gas, which was widely used before the 1960s for domestic lighting, cooking, and heating, had carbon monoxide as a significant constituent. Iron smelting and other current technological processes still produce byproduct carbon monoxide.[2]
Worldwide, the largest source of carbon monoxide is from the photochemical reactions in the troposphere that generate about 5 x 1012 kilograms per year.[3] Other natural sources of CO include volcanoes, forest fires, and other forms of combustion.
Toxicity
Carbon monoxide poisoning is the most common type of fatal air poisoning in many countries. It combines with hemoglobin in the red blood cells of humans and animals to produce carboxy hemoglobin, which is ineffective for delivering oxygen to bodily tissues. Exposure to carbon monoxide levels as low as 667 parts per million by volume (ppmv) may cause up to 50 % of the body's hemoglobin to convert to carboxyhemoglobin[4] which may result in seizure, coma, and fatality.
On average, exposures to levels of 100 ppm or greater is dangerous to human health. In the United States, the long-term workplace exposure levels are limited by the Occupational Safety and Health Administration (OSHA) to less than 50 ppmv averaged over an 8-hour period;[5] in addition, employees are to be removed from any confined space if an upper limit (ceiling) of 100 ppmv is reached.
The most common symptoms of carbon monoxide poisoning may resemble other types of poisonings and infections, including symptoms such as headache, nausea, vomiting, dizziness, fatigue, and a feeling of weakness. Infants may be irritable and feed poorly.[6] Neurological signs include confusion, disorientation, visual disturbance, fainting and seizures. (See adjacent table.)
Exposures to carbon monoxide may cause significant damage to the heart and central nervous system, especially to a sub-cortical component of the brain, often with long-term effects. Carbon monoxide may have severe adverse effects on the fetus of a pregnant woman.
Occurrence
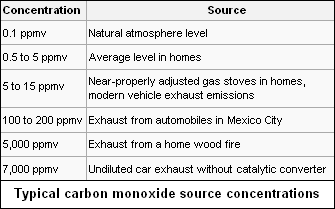
Carbon monoxide occurs in various natural and artificial environments. Typical concentrations in parts per million by volume are as follows:[7][8][9]
Atmosphere
Carbon monoxide is present in low levels in the atmosphere, primarily as a product of volcanic activity but also from natural and man-made fires such as slash and burn agriculture, burning of crop residues, and sugarcane fire-cleaning. Combustion of fossil fuels also results in carbon monoxide emissions to the atmosphere.
Through natural processes in the atmosphere, carbon monoxide is eventually converted to carbon dioxide. Carbon monoxide concentrations in the atmosphere are short-lived and geographically variable.