Greenhouse effect
This article is a stub and is being actively worked on.
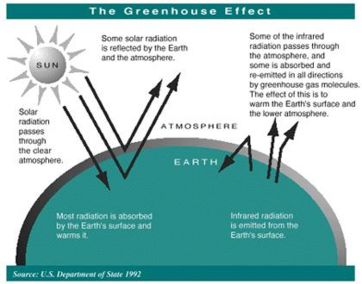
The sun radiates energy to the earth mainly in the form of ultraviolet and visible (short wavelength) light. This energy, which is not absorbed by a clear atmosphere, warms the surface the earth. The warm surface radiates energy back into space in the form of infrared (long wavelength) light. Much of the infrared energy radiated by the surface does not disappear in space, but is absorbed by greenhouse gases present in the lower atmosphere. This absorption warms the lower atmosphere.
The greenhouse effect (or "atmospheric effect") is a general attribute of planets and moons with atmospheres. It is an imbalance between surface radiation and top-of-atmosphere radiation due to the presence of greenhouse gases. For example, in the case of the Earth, the surface emits 390 W/m2[1] (averaged over a year and the whole surface), but the emission at the top of the atmosphere is 235 W/m2, giving a global-average greenhouse effect of 155 W/m2[2]. The top-of-atmosphere outgoing radiation balances the incoming 235 W/m2 of solar radiation (342 W/m2 incident minus 107 W/m2 reflected). The term "greenhouse effect" is a misnomer, since actual greenhouses operate by a different mechanism.
The greenhouse and the planets
The physics of the greenhouse
The essential condition for a greenhouse effect is the presence in a planetary atmosphere of gases that absorb (and emit) in the thermal radiation band of the planetary surface, or lower atmospheric levels. Frequently, the greenhouse gases will be transparent, or nearly so, to incoming solar radiation (ozone is an exception). The surface thermal radiation band will be in the long-wave infrared region (3.5 µm - 100 µm). The greenhouse gases are triatomic (or more, but note the exception for Titan) molecules which absorb energy into a variety of rotational, bending, and stretching modes. Common solar-system gases that meet the requirements are water vapor (H2O), carbon dioxide (CO2), ozone (O3), and methane (CH4). For Earth, H2O, CO2, and O3 provide most of the greenhouse. Common homonuclear diatomic gases, such as nitrogen (N2) and oxygen (O2), do not afford dipole transitions in the infrared and hence hardly absorb energy.
The greenhouse effect occurs at a state near local thermodynamic equilibrium. When a gas molecule is excited by an infrared quantum, the energy is promptly redistributed, by collisions, among the available degrees of freedom (of all gases present) according to the principles of statistical mechanics. So infrared absorption adds energy directly to the local thermal reservoir. Likewise, thermal infrared emission withdraws energy. In simple terms, this means that an atmosphere with greenhouse gases present absorbs heat by absorbing radiation of the proper frequency as it passes through, and loses heat by emitting the same frequencies. Whether the temperature rises or falls depends on the balance between the two effects and on any other heating or cooling processes. It should be noted that the greenhouse gas molecules maintain their identity, and are not destroyed or chemically altered. This differs from the case of ionizing radiation absorption (such as the absorption of solar ultraviolet radiation by oxygen and ozone).
References and notes
External links
Additional bibliography
- Houghton, JT, 1977. The Physics of Atmospheres, 3rd Ed, Cambridge Univ. Press.
- Peixoto, JP, and AH Oort, 1992. The Physics of Climate, American Institute of Physics.
- Thomas, GE, and K Stamnes, 1999. Radiative Transfer in the Atmosphere and Ocean, Cambridge Univ. Press.