Toxoplasma gondii
| Toxoplasma gondii | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Toxoplasma gondii |
Toxoplasma gondii is a single celled organism that is known for being the cause of toxoplasmosis, a common food born illness. T. gondii is a unicellular, parasitic protist, classified as an apicomplexan within the group alveolate. Apicomplexans are exclusively parasitic organisms and derive their name from their apical complex. T. gondii is a close relative of plasmodium, the parasite that causes malaria. Like plasmodium they share structural characteristics, most importantly specialized organelles in the apical end. Unlike plasmodium however, T. gondii is able to survive outside the liver cells and red blood cell. In fact, T. gondii is able to inhabit any cell in the body. One study found that nearly one quarter of adults and adolescents in the United States have been infected with T. gondii[1]. And while many of those infected with T. gondii do not display symptoms, individuals with compromised immune systems such as those infected with the AIDS virus have an increased risk of death due to Toxoplasomosis.
Structure, Multiplication, and Life Cycle
Cell Structure
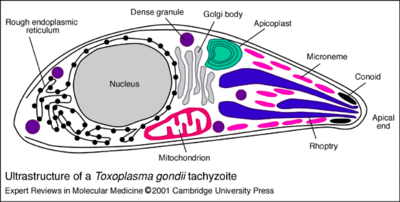
T. gondii is a unicellular protist with an amorphous amoeboid-like body form. They have no flagella or other motility structures but move by amoeboid motion. The plasma membrane is surrounded by a cell wall. Although they do not have a contractile vesicle they have alveoli just below their plasma membrane. These alveoli are subcellular cavities characteristic of many protists that are important for stabilization of the cellular surface. The apical end of their spores, as can be seen from the image below, contains a mass of organelles that help the spores invade their host tissue. [2]
Genome structure
The complete circular T. gondii genome was sequenced by The University of Pennsylvania in 2006 using the Random Shot-gun method. The genome is a circular chromosome that consists of 34,996 base pairs and has a GC content of 21%. Of the entire genome, fifty two percent codes for proteins. Of the 63 genes present, 37 encode structural RNAs and 26 code for other proteins. [3]
Use the following link to view the complete genome of T. gondii.
[1]
Life cycle
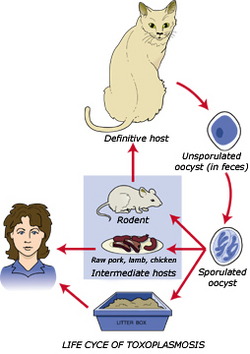
Much like its close relative plasmodium, toxoplasma gondii has a complex life cycle that alternates between sexual and asexual stages. The various stages of its life cycle take place in different organisms, cycling between cats and the animals they eat. The cat releases T. gondii oocysts in its feces, which get picked up by ingestion by another animal, generally known as the intermediate host. Once the oocysts enter this new host they multiply to 128 copies and hatch, allowing the parasite to move through the hosts body and attack new cells. After a few days inside the intermediate host however, the organism de-differentiates and becomes simpler in morphology in a process called encystment. The cyst that forms contains many individual T. gondii cells, and is marked by the presence of a cell wall and a low metabolic rate. Cyst formation is important for a few reasons. Most significantly they serve as a means of transfer between the host and the parasitic species. Additionally formation of a cyst will protect the parasite from any unfavorable environmental conditions such as nutrient deficiency, desiccation, and extreme pH's. [2]
The parasite enters the human circulatory system when these cysts are ingested by humans. Toxoplasmosis can be contracted in one of three ways. Eating raw or undercooked meat containing the parasite T. gondii is the most common way to a person becomes infected. Ingesting T. gondii oocysts from soil through gardening, handling or eating unwashed vegetables, or changing a cat litter box is another common form of contraction. Pregnant women who come in contact with T. gondii can also pass along their acquired infection to their fetus through the placenta. Once inside their final host they undergo a process known as excystment, where the parasite escapes from the cyst and begins to divide once again. This is generally triggered by the return of favorable environmental conditions. [4]
Ecology
T. gondii need moisture to avoid desiccation, and therefore take up residence in mammalian body fluids. They are exclusively parasitic and therefore do not survive outside a host's body. [5]
Pathology
Toxoplasma gondii is responsible for the disease Toxoplasmosis. Toxoplasma infects a host but keeps the host alive so it can use their immune system and thereby keep itself alive. It does this by releasing a molecule that increases the number of T cells. An increased number of T-cells results in a massive elimination of Toxoplasma gondii cells. Parasitic cells that are encased in a tougher outer cyst are able to evade destruction by the immune systems macrophages. These cysts remain dormant in the host’s body and do not confer much damage. From time to time however, the parasite will rupture from the cyst and reactivate the host’s immune system. Once activate the host may experience some mild symptoms, until the parasite is forced back into their cysts by the host’s immune system once again. [4]
Toxoplasma gondii only becomes a threat to humans when the host immune system is unable to control any ruptured cyst. Fetuses that don’t have fully developed immune systems, and individuals with weakened immune systems are unable to protect themselves from T. gondii infection. When toxoplasma cysts erupt in a host with AIDS, a strong immune response is required to drive the parasites back into their cysts. Without an immune system to keep it in check, Toxoplasma gondii will replicated uncontrollably, often leading to illness or death. [4]
Symptoms
When a person becomes infected with T. gondii they may experience mild with flu-like symptoms that last for several weeks and then go away, such as tender lymph nodes, and muscle aches. A healthy individual however, may not have symptoms because their immune system can keep the parasite from causing illness. According to Carl Zimmer one third of all people living on this world are infected with T. gondii even though few are aware about it. People with compromised immune systems, such as individuals with HIV have a much higher risk for developing severe signs of toxoplasmosis, and may experience severe symptoms such as fever, confusion, headache, seizures, nausea, and poor coordination. Toxoplasmosis is generally not passed from person-to-person, but can be passed from mother to child during pregnancy. Infection of the fetus can lead to miscarriage, a stillborn child, or a child born with abnormal enlargement or smallness of the head. Infants infected before birth often show no symptoms at birth but develop them later in life with potential vision loss, mental disability, and seizures.
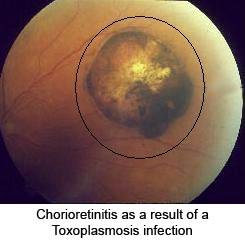
Another common manifestation of toxoplasmosis is retinochoroiditis, or eye disease. These eye infections can lead to acute inflammatory lesions of the retina. Associated symptoms are eye pain, sensitivity to light or photophobia, tearing of the eyes, and blurred vision. Eventual blindness will result if the central structures of the retina are infected by the parasite.[6]
Additionally T. gondii appears to manipulate personality of the infected by the same adaptations that normally help it complete its life cycle. The typical life cycle of the parasite involves a cat and its prey. The cycle begins when T. gondii eggs are shed in an infected cat's feces, which are then inadvertently eaten by a warm-blooded animal, such as a rat. Toxoplasma gondii can change a rats' behavior by altering neurtransmitter function. The infected rat's behavior alters so that it becomes more active, less cautious and more likely to be eaten by a cat, where the parasite completes its life cycle. Many other warm-blooded vertebrates may be infected by this parasite, which can in turn effect their personalities as well. After producing usually mild flu-like symptoms in humans, the parasite tends to remain in a dormant state in the brain and other tissues. Toxoplasma gondii has been implicated in a in a wide variety of personality disorders as well in humans as well. [4]
Diagnosis
Toxoplasmosis can be diagnosed by various means. The most common way to determine the presence of T. gondii is by serological testing, where immunoglobulin G (IgG) levels in the blood are measured. This is used to determine the presence of T. gondii in the individual being tested. Serological testing is the main form of diagnosis of toxoplasmosis, and is commonly used in population studies of toxoplasmosis. Another, more direct form of diagnosis is through the staining of body fluid samples such as blood or cerebrospinal fluid. This method is not used as frequently as serological testing due to various technical difficulties. Various molecular techniques are being developed to detect the presence of the parasite's DNA in the affected body fluid. These procedures can be useful in identifying the presence of T. gondii in the fetus by testing the amniotic fluid. [6]
Treatment
Most healthy people are able to recover from the various flu-like symptoms of toxoplasmosis without treatment. The parasite however, may remain within the host cells in a less active phase. If a person were to become sick they can be treated with drugs such as pyrimethamine or sulfadiazine, and folinic acid. Additionally, ocular lesions caused by toxoplasmosis can be treated by various means. Treatment of patients with compromised immune systems is more complicated for they may need long-term treatment as long as they are immunosuppressed.[6]
Current Research
Much of the research being done T. gondii looks at the connection between toxoplasmosis and HIV. This sort of research is important because nearly 60% of deaths in patients with AIDS are a direct result of infection other than HIV, one of which is toxoplasmosis.
A case study was done by Sharenda Williams and Elizabeth Burton on toxoplasmosis in a patient with undiagnosed AIDS. The case involved a 44-year-old Hispanic man who presented with fever, chills, cough, weight loss, abdominal pain, nausea and vomiting, diarrhea, headache, eye pain, and fatigue. The patient underwent an extensive infectious workup, including tests for various organisms other than T. gondii. Although treated with antibiotics including levofloxacin, metronidazole, doxycycline, and caspofungin for presumed sepsis the patients symptoms persisted and he died after 4 days in the hospital. An autopsy revealed Toxoplasma gondii cysts (bradyzoites) and free tachyzoites (trophozoites) in the heart, lungs, brain, adrenal glands, appendix, stomach, pancreas, bladder, right testis, spleen, gallbladder, lymph nodes, muscle, and bone marrow upon microscopic analysis. In this case the presence of T. gondii was only determined post-mortem.[7]
A new method of detection and diagnosis of toxoplasmosis in patients with AIDS is being researched by Yenisey Alfonso et al. Their paper discusses the molecular diagnosis of Toxoplasma gondii infection in cerebrospinal fluid from AIDS patients. The aim of this study was to evaluate a rapid PCR method using the B1 gene to detect T. gondii in CSF samples from patients with suspected Toxoplasmic encephalitis. TE is one of the most common infections in immunocompromised patients, and is the main cause of cerebral lesions in HIV patients. The detection of Toxoplasma gondii by PCR may facilitate the diagnosis of TE in patients with AIDS. In this study diagnosis is accomplished by direct identification of the parasite DNA in tissue samples. The results of this study showed that using the B1 gene and B22/B23 set of primers in PCRs is a rapid and reliable method for determining the present of Toxoplasma gondii in an individual. Additionally this procedure can be used to differentiate between toxoplasmosis and other central nervous system (CNS) diseases.[8]
References
- ↑ Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB (2001). "Toxoplasma gondii infection in the United States: seroprevalence and risk factors". Am J Epidemiol 154 (4): 357-65. DOI:10.1093/aje/154.4.357. PMID 11495859. Research Blogging. [e]
- A large-scale survey on the prevalence of Toxoplasma gondii across the United States which also contains analyses of correlation with a number of other variables. For example, the correlation was found to be notable for diet and environment, weaker for cat ownership.
- ↑ 2.0 2.1 [Heller Craig, Orians Gordon, Purves William, Sadava David. 2004. Life: The Science of Biology 7th Ed.]
- ↑ Media:http://www.ncbi.nlm.nih.gov/sites/entrez?Db=genome&Cmd=ShowDetailView&TermToSearch=12164
- ↑ 4.0 4.1 4.2 4.3 [Carl Zimmer. 2001. Parasite Rex: Inside the Bizarre World of Nature's Most Dangerous Creatures. Simon and Schuster]
- ↑ [Sherwood Linda, Willey Joanne, Woolverton Christopher. 2008. Microbiology 7th Ed. McGraw Hill: US ]
- ↑ 6.0 6.1 6.2 Media:http://www.cdc.gov/toxoplasmosis/
- ↑ Sharenda L. Williams, MD and Elizabeth C. Burton, MD. 2009. Disseminated toxoplasmosis in a patient with undiagnosed AIDS. Baylor University Medical Center Proceedings. Vol. 22
- ↑ Yenisey Alfonso,1 Jorge Fraga, 1 Carlos Fonseca,2 Narciso Jiménez,2 Taimy Pinillos,2 Alberto J Dorta-Contreras,3 Raymundo Cox,1 Virginia Capó,2 Olga Pomier,2 Francisco Bandera,2 and Dora Ginorio1. 2009. Molecular diagnosis of Toxoplasma gondii infection in cerebrospinal fluid from AIDS patients. Cerebrospinal Fluid Research. Vol. 6