Lipostatic hypothesis
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is 01 April 2012. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
In 1953, Kennedy [1] proposed what became known as the lipostatic hyothesis. Specifically, he postulated the existence, in the hypothalamus, of a centre that was sensitive to the concentration of metabolites in the circulation, which prevented "an overall surplus of energy intake over expenditure."
Introduction
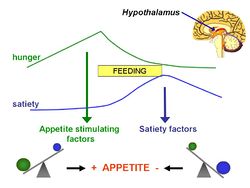
The lipostatic hypothesis describes the feedback mechanisms between adipose tissue deposition and hypothalamic signalling, in order to sustain 'lipostasis'. Lipostasis is achieved through the modulation and balancing of energy intake and expenditure to prevent an individual 'getting fat'. For the brain to know when their is too much adipose tissue building up, a signalling hormone must be released from the adipose tissue to signal to the brain that a satiation signal must be released in order to stop the individual from eating. In 1994, it was discovered that this hormone released from adipose tissue, and critical in regulating the size of the body fat depot, was coded for by the ob gene and is known as leptin.
What is leptin?
Hypothalamic leptin signaling
Leptin receptor mediated hypothalamic signaling involves multiple pathways. The JAK2/cytosolic signal transducer and activator of transcription protein 3 (STAT3) pathway plays an important role, although the JAK2-Phosphotidylinosital 3 kinase (PI3K) pathway has also shown to be important. With respect to the JAK2/STAT3 pathway, leptin binding to the long form of the leptin receptor (Ob-Rb) initiates tyrosine phosphorylation of Ob-Rb by JAK2. Phosphorylated leptin receptor then recruits STAT3 that is activated through phosphorylation by JAK2. The activated STAT3 proteins dimerize and translocate to the nucleus where they bind DNA and initiate gene transcription. Suppresser of cytokine signaling 3 (SOCS-3) and phosphotyrosine phosphotase 1B (PTP1B) are two known negative regulators of leptin signaling following Ob-Rb activation. The JAK2/STAT3 pathway is believed to be essential for mediating leptin's effects on homeostatic energy regulation.
Parabolis evidence
Conclusion
References
Zhang Y & Scarpace PJ (2006) The role of leptin in leptin resistance and obesity. Physiol Behav. 88, 249-256.