Melanocortins and appetite
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is 01 February 2011. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
Melanocortins and appetite
Overview of Pro-opiomelanocortin (POMC)
Alpha melanocyte-stimulating hormones
Melanocortin Receptors
The Melanocortin Pathway
The control and regulation of feeding involves a complex interplay between a number of circulating hormones, neurotransmitters and nutrients, and the Melanocortin system has been identified as being a central component. The melanocortin system is the name collectively given for; ►Neurons arising in the arcuate nucleus of the hypothalamus, and express AgRP,NPY or POMC. ►POMC neurons that project to the brainstem ►Melanocortin receptors, predominately MC3R and MC4R that respond to POMC peptides and AgRP. In addition to its involevement in regulating energy homeostasis, the Melancortin system plays a role in mediating a number of physiological processes within the body including (3)
The control and regulation of feeding involves a complex interplay between a number of circulating hormones, neurotransmitters and nutrients, and POMC containing neurons have been identified at the point where these interactions occur.
Although the Melanocortin system is known to be central to the regulatory mechanisms controlling appetite and satiety, the precise mechanism is not fully understood. Complexity arises from both the direct and indirect effects of a number of compounds including leptin, insulin, glucose, ghrelin, NPY, serotonin, peptide YY and endorphin, all of which act in isolation and in sync to mediate their effects on these POMC neurons.
Below is a table showing the opposing effects that POMC agonists and antagonists have on feeding behaviour.
Agonists Antagonists Reduce food intake Enhance food intake (hyperphagia) Increase energy expenditure Decrease energy expenditure Reduce body weight Increase body weight
This highlights the role of the melanocortin system in regulating energy homeostasis, and why disruption in the genes controlling this system, i.e. genetic mutations of the system, can result in individuals which are hyperphagic and consequentially obese.
A number of mutations of this system have been identified in mice, all of which show a dysregulation in energy homeostasis. Many of the mutations discovered involve excess production of POMC antagonists, so that POMC agonists can’t bind to POMC receptors in order to suppress appetite.
1) Excess production of the agouti protein induces its’ antagonistic effects through binding to both the MC1R and MC4R. 2) An increase in the expression of AGRP which functions by antagonising receptors MC3R and MC4R. This prevents the potent appetite suppressor alpha MSH from binding. 3) A mutation which results in a deficiency in the number of MC4R receptors. 4) The insufficient production of POMC derived peptides to bind to these receptors. Obese individuals have presented with POMC deleterious gene mutations, as well heterozygous mutations in the MC4R receptor. .
POMC neurons and their peptides mediate satiety signals, while NPY neurons induce hunger signals with decreased energy expenditure. The median eminence of the arcuate nucleus receives projections from both POMC and NPY neurons, highlighting its role in controlling both energy expenditure as well as hunger/satiety signals. This integration involves both long term signals (leptin from adipose tissue and insulin), as well as acute hunger/satiety signals from the brainstem.
The melancortin Alpha MSH has been identified as one of the most important regulators of energy homeostasis in the hypothalamus, where it induces a state of satiety within an individual through its actions on the MC4R. Additionally, administration of ACTH into certain regions of the hypothalamus has similar effects. Agouti is an antagonist at MC1R and MC4R receptors, while AGRP incurs antagonistic effects through its action on MC3R and MC4R receptors. Due to suppression of the alpha MSH anorectic signal, mutant mice with ectopic expression of these peptides are hyperphagic with an increase in adipose mass, lean mass, hyperinsulinemia and consequentially are clinically obese.
Role of Leptin and Insulin
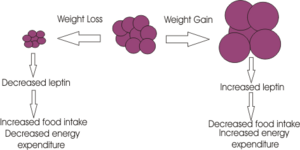
Leptin released form adipose tissue and insulin from the pancreas, also serve to regulate food intake. Excess adipose tissue (i.e. as occurs in obese individuals) results in an increase in leptin production (which normally induces a feeling of satiety), but excess production of NPY peptides (as occurs in some mutations of the melancortin system), can suppress its effects. Similarly insulin levels show a marked increase in obese individuals, with hyperinsuliemia being one of the first metabolic disturbances identified in those obese subjects with mutations of the melanocortin system.
Ghrelin
The role of the potent appetite stimulant within the melanocortin system has been verified, whereby central administration of this peptide results in excessive eating, but if NPY antagonists are given in conjunction,a state of hyperphagia is not induced. During periods of meal deprivation, Ghrelin levels increase. They induce their potent appetite stimulating properties by activating arcuate NPY and AgRP expression. In contrast, following food consumption, ghrlein levels show a marked decrease.
An example of the complexity that arises in fully understanding the precise mechanism governing this system arises from mice AgRP KO models. Interestingly, while the involvement of AgRP in the melanocortin system is undisputed, one would expect such KO to present with altered phenotypes and eating patterns, yet these AgRP were just like their wild type counterparts.
Although Peptide YY has been identified as an appetite suppressor, it appears to mediate its effects through a distinct pathway that does not involve the melanocortin system as both POMC and MC4R KO continued to show a decrease in food intake following its administration, indicating that another system may be involved. This further highlights the complexity of the mechanisms controlling energy homeostasis.
Animal models and human examples of defects in the melanocortin system
Experimental evidence and methods used to investigate melanocortin
Theories for melanocortin's role in appetite and suggested future studies
Discussion
Figures and Diagrams
You can also insert diagrams or photographs (to Upload files Cz:Upload)). These must be your own original work - and you will therefore be the copyright holder; of course they may be based on or adapted from diagrams produced by others - in which case this must be declared clearly, and the source of the orinal idea must be cited. When you insert a figure or diagram into your article you will be asked to fill out a form in which you declare that you are the copyright holder and that you are willing to allow your work to be freely used by others - choose the "Release to the Public Domain" option when you come to that page of the form.
When you upload your file, give it a short descriptive name, like "Adipocyte.png". Then, if you type {{Image|Adipocyte.png|right|300px|}} in your article, the image will appear on the right hand side.
Begin your article with a brief overview of the scope of the article on interest group. Include the article name in bold in the first sentence.[1]
Remember you are writing an encyclopedia article; it is meant to be readable by a wide audience, and so you will need to explain some things clearly, without using unneccessary jargon. But you don't need to explain everything - you can link specialist terms to other articles about them - for example adipocyte or leptin simply by enclosing the word in double square brackets.
You can write your article directly onto the wiki- but at first you'll find it easier to write it in Word and copy and paste it onto the wiki.
Construct your article in sections and subsections, with headings and subheadings like this:
References
To insert references and/or footnotes in an article, put the material you want in the reference or footnote between <ref> and </ref>, like this:
<ref>Person A ''et al.''(2010) The perfect reference for subpart 1 ''J Neuroendocrinol'' 36:36-52</ref> <ref>Author A, Author B (2009) Another perfect reference ''J Neuroendocrinol'' 25:262-9</ref>.
Look at the reference list below to see how this will look.[2] [3]
If there are more than two authors just put the first author followed by et al. (Person A at al. (2010) etc.)
Select your references carefully - make sure they are cited accurately, and pay attention to the precise formatting style of the references. Your references should be available on PubMed and so will have a PubMed number. (for example PMID: 17011504) Writing this without the colon, (i.e. just writing PMID 17011504) will automatically insert a link to the abstract on PubMed (see the reference to Johnsone et al. in the list.)
[4]
Use references sparingly; there's no need to reference every single point, and often a good review will cover several points. However sometimes you will need to use the same reference more than once.
How to write the same reference twice:
Reference: Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431 PMID 17072591
First time: <ref name=Berridge07>Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. ''Psychopharmacology'' 191:391–431 PMID 17072591 </ref>
Second time:<ref name=Berridge07/>
This will appear like this the first time [5] and like this the second time [5]
- ↑ See the "Writing an Encyclopedia Article" handout for more details.
- ↑ Person A et al. (2010) The perfect reference for subpart 1 J Neuroendocrinol 36:36-52
- ↑ Author A, Author B (2009) Another perfect reference J Neuroendocrinol 25:262-9
- ↑ Johnstone LE et al. (2006)Neuronal activation in the hypothalamus and brainstem during feeding in rats Cell Metab 2006 4:313-21. PMID 17011504
- ↑ 5.0 5.1 Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431 PMID 17072591