User:Milton Beychok/Sandbox
The Claus process is a catalytic chemical process for converting gaseous hydrogen sulfide (H2S) into elemental sulfur.[1] The process is commonly referred to as a sulfur recovery unit (SRU) and is very widely used to produce sulfur from the hydrogen sulfide found in raw natural gas and from the by-product gases containing hydrogen sulfide derived from refining petroleum crude oil and other industrial facilities.
There are many hundreds of Claus sulfur recovery units in operation worldwide. In fact, the vast majority of the 66,000,000 metric tons of sulfur produced worldwide in 2006 was by-product sulfur from petroleum refining and natural gas processing plants.[2][3]
Feed gas composition
Claus unit feed gases have a wide range of compositions. Most of the feed gases are originate from absorption processes using various solvents to extract hydrogen sulfide from the by-product gases of petroleum refining, natural gas processing, tar sands processing, coal gasification and other industries. The absorption processes used for that purpose include amine gas treating, Rectisol and Selexol to name a few.
In addition to hydrogen sulfide extracted from by-product gases by an absorption process, petroleum refineries also derive hydrogen sulfide from the steam distillation of wastewaters containing dissolved hydrogen sulfide. Those wastewaters are referred to as sour water and the steam distillation process is referred to as sour water stripping.
The table below provides a typical analysis of the Claus feed gases obtained from amine gas treating and from sour water stripping:
| Component | Mol % | Wt % | Component | Mol % | Wt % |
|---|---|---|---|---|---|
| From an amine process: | From a sour water stripper: | ||||
| H2S | 82.1 | 80.8 | H2S | 26.7 | 40.2 |
| CO2 | 11.9 | 15.1 | CO2 | 2.6 | 5.1 |
| NH3 | nil | nil | NH3 | 39.4 | 29.7 |
| H2O | 4.0 | 2.1 | H2O | 31.3 | 25.0 |
| HC | 2.0 | 2.0 | HC | nil | nil |
Gases with an H2S content of over 25% are suitable for the recovery of sulfur in straight-through Claus plants while alternate configurations such as a split-flow set up or feed and air preheating can be used to process leaner feeds.[4]
The amount of hydrogen sulfide derived from sour water sripping in a petroleum refinery is very much less than than is derived from the refinery's sour water stripper.
Process flow diagram and description
The Claus reaction to convert H2 into elemental sulfur requires the presence of one mole of SO2 for each two moles of H2S:
- 2H2S + SO2 → 3S + 2H2O
To provide that ratio of components, the first step in the Claus process is the combustion of one-third of the H2S in the feed gas:
- H2S + 1.5 O2 → SO2 + H2O
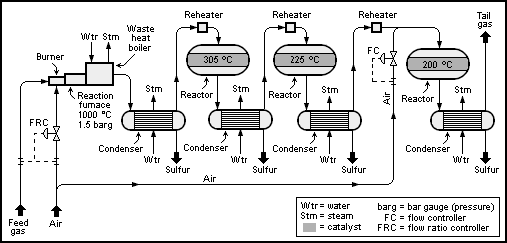
Thus, the feed gas is fed into a fire-tube boiler (or other special type of gas-fired boiler) along with sufficient air to burn only one-third of the H2S it contains. This is done with a flow ratio controller as shown in the schematic process flow diagram below:
The Claus technology can be divided into two process steps, thermal and catalytic.
In the thermal step, hydrogen sulfide-laden gas reacts in a substoichiometric combustion at temperatures above 850 °C such that elemental sulfur precipitates in the downstream process gas cooler.
The H2S-content and the concentration of other combustible components (hydrocarbons or ammonia) determine the location where the feed gas is burned. Claus gases (acid gas) with no further combustible contents apart from H2S are burned in lances surrounding a central muffle by the following chemical reaction:
Gases containing ammonia, such as the gas from the refinery's sour water stripper (SWS), or hydrocarbons are converted in the burner muffle. Sufficient air is injected into the muffle for the complete combustion of all hydrocarbons and ammonia. Air to the acid gas is controlled such that in total 1/3 of all hydrogen sulfide (H2S) is converted to SO2. This ensures a stoichiometric reaction for the Claus reaction (see next section below).
The separation of the combustion processes ensures an accurate dosage of the required air volume needed as a function of the feed gas composition. To reduce the process gas volume or obtain higher combustion temperatures, the air requirement can also be covered by injecting pure oxygen. Several technologies utilizing high-level and low-level oxygen enrichment are available in industry, which requires the use of a special burner in the reaction furnace for this process option.
Usually, 60 to 70% of the total amount of elemental sulfur produced in the process are obtained in the thermal process step.
The main portion of the hot gas from the combustion chamber flows through the tube of the process gas cooler and is cooled down such that the sulfur formed in the reaction step condenses. The heat given off by the process gas and the condensation heat evolved are utilized to produce medium or low-pressure steam. The condensed sulfur is removed at the gas outlet section of the process gas cooler.
A small portion of the process gas can be routed through a bypass inside of the process gas cooler, as depicted in the figure. This hot bypass stream is added to the cold process gas through a three-way valve to adjust the inlet temperature required for the first reactor.
Catalytic step
The Claus reaction continues in the catalytic step with activated alumina or titanium dioxide, and serves to boost the sulfur yield. The hydrogen sulfide (H2S) reacts with the SO2 formed during combustion in the reaction furnace, and results in gaseous, elemental sulfur. This is called the Claus reaction:
- 2H2S + SO2 → 3S + 2H2O
The catalytic recovery of sulfur consists of three substeps: heating, catalytic reaction and cooling plus condensation. These three steps are normally repeated a maximum of three times. Where an incineration or tail-gas treatment unit (TGTU) is added downstream of the Claus plant, only two catalytic stages are usually installed.
The first process step in the catalytic stage is the process gas heating. It is necessary to prevent sulfur condensation in the catalyst bed, which can lead to catalyst fouling. The required bed operating temperature in the individual catalytic stages is achieved by heating the process gas in a reheater until the desired operating bed temperature is reached.
Several methods of reheating are used in industry:
- Hotgas bypass: which involves mixing the two process gas streams from the process gas cooler (cold gas) and the bypass (hot gas) from the first pass of the Wasteheat boiler.
- Indirect Steam reheaters: the gas can also be heated with high pressure steam in a heat exchanger.
- Gas/Gas Exchangers: whereby the cooled gas from the process gas cooler is indirectly heated from the hot gas coming out of an upstream catalytic reactor in a gas-to-gas exchanger.
- Direct-fired Heaters: fired reheaters utilizing acid gas or fuel gas, which is burned substoichiometrically to avoid oxygen breakthrough which can damage Claus catalyst.
The typically recommended operating temperature of the first catalyst stage is 315°C to 330°C (bottom bed temperature). The high temperature in the first stage also helps to hydrolyze COS and CS2, which is formed in the furnace and would not otherwise be converted in the modified Claus process.
The catalytic conversion is maximized at lower temperatures, but care must be taken to ensure that each bed is operated above the dewpoint of sulfur. The operating temperatures of the subsequent catalytic stages are typically 240°C for the second stage and 200°C for the third stage (bottom bed temperatures).
In the sulfur condenser, the process gas coming from the catalytic reactor is cooled to between 150 and 130°C. The condensation heat is used to generate steam at the shell side of the condenser.
Before storage, liquid sulfur streams from the process gas cooler, the sulfur condensers and from the final sulfur separator are routed to the degassing unit, where the gases (primarily H2S) dissolved in the sulfur are removed.
The tail gas from the Claus process still containing combustible components and sulfur compounds (H2S, H2 and CO) is either burned in an incineration unit or further desulfurized in a downstream tail gas treatment unit.
Gases with an H2S content of over 25% are suitable for the recovery of sulfur in straight-through Claus plants while alternate configurations such as a split-flow set up or feed and air preheating can be used to process leaner feeds.[5]
Process performance
Using two catalytic stages, the process will typically yield over 97% of the sulfur in the input stream. Over 2.6 tons of steam will be generated for each ton of sulfur yield.
History
First invented over 100 years ago, the Claus process has become the industry standard.
The process was invented by Carl Friedrich Claus, a chemist working in England. A British patent was issued to him in 1883. The process was later significantly modified by a German company called I.G.Farbenindustrie A.G.[6]
References
- ↑ Gary, J.H. and Handwerk, G.E. (1984). Petroleum Refining Technology and Economics, 2nd Edition. Marcel Dekker, Inc.. 0824771508.
- ↑ Sulfur production report by the United States Geological Survey
- ↑ Discussion of recovered byproduct sulfur
- ↑ Gas Processors Association Data Book, 10th Edition, Volume II, Section 22
- ↑ Gas Processors Association Data Book, 10th Edition, Volume II, Section 22
- ↑ Bibliographic Citation