Steroid
Steroids, or steroid hormones, are powerful hormones with drastic effects, both good and bad, when artificially supplimented into living systems. They play a role in all stages of life from the embryo until death. Corticosteriods are widely used to treat asthma patients, but do have severe side effects. Athletes have often taken anobolic steroids to improve muscle growth and athletic performance. Glucocorticoids play a role in inflamation, and estrogens have been linked to cancer. Testosterone and estrogen influence sexual traits (maleness/femaleness). All steroid hormones are naturally synthesized from cholesterol (through pregnenolone) under the control of the anterior pituitary gland, which produces andrenocorticotropic hormone (ACTH, or corticotropin), a polypeptide that stimulates the conversion of cholesterol to pregnenolone. The five major classes of steroids are: progestagens, glucocorticoids, mineralcorticoids, androgens and estrogens. The steroid hormones activate gene expression by binding to enhancer proteins, called steroid receptors. Deficiency of the enzyme 21-hydroxylase is the most common steroid-related in-born metabolic disorder, but treatments are available for this condition.
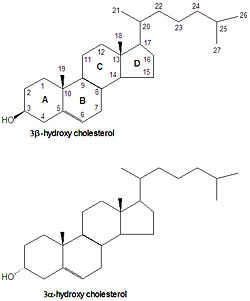
cholesterol
Cholesterol is the precursor from which all steroid hormones are synthesized. Because of this, all steroid numbering and nomenclature follow that of cholesterol. Some cholesterol derivatives have a proton added to the C-5 carbon. If the H-5 proton is -oriented (points down) then rings A and B are fused in a trans conformation, but in the <math?\beta</math> orientation, the rings are fused in a cis conformation. All steroids with an H5 are in the 5 orientation, while bile salts derived from cholesterol have the opposite 5 orientation.
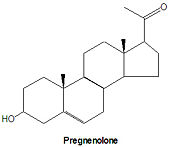
pregnenolone and progestagens
All steroid hormones have 21 or fewer carbons, although their precursor chemical, cholesterol, has 27 carbon atoms. Pregnenolone is the first steroid derived from cholesterol. It is synthesized through an intermediate, 20,22-dihydroxy-cholesterol, which is subsequently oxidized at C-20 to form a ketone with cleavage of carbons 22-27.
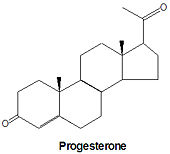
Progesterone, a progestagen which prepares the lining of the uterus for implantation of an ovum, is biosynthesized from prognenolone by oxidation of the 3-hydroxy group into a 3-keto group and by the isomerization of the 5 double bond into a 4 double bond. This hormone is also essential to maintain pregnancy. Progresterone is the precursor chemical in the biosynthesis of corticoids and androgens.
glucocorticoids and mineralcorticoids
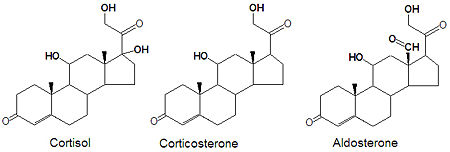
Glucocorticoids and mineralocorticoids (or mineralcorticoids) are naturally synthesized by the enzymatic oxidation of progesterone. Cortisol is produced when progesterone is hydroxylated at three positions, C-11, C-17 and C21. The oxidation of the C-17 carbon must occur before the hydroxylation at C-21 to synthesize cortisol, otherwise corticosterone is formed. Aldosterone, the major mineralcorticoid, is synthesized from corticosterone by oxidation of the C-18 methyl group to form an aldehyde.
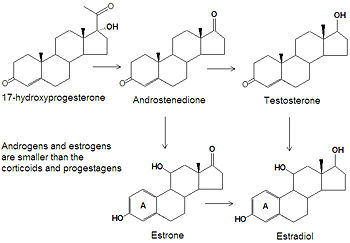
androgens and estrogens
While the progestagens, glucocorticoids and mineralcorticoids all have twenty one carbon atoms, the smaller androgens and estrogens have only nineteen or eighteen carbon atoms, respectively. The biosynthesis of both classes of steroids starts with the hydroxylation of progesterone at C-17 to produce 17<math\alpha</math>-hydroxyprogesterone. Cleavage of the C20-C21 side chain and oxidation of the C-17 hydroxyl group into a ketone produces androstenedione. Reduction of the C-17 keto group of androstenedione yields testosterone, one of the most abused anabolic steroids. The smallest class of steroids, the estrogens, are biosynthesized from the androgens by loss of the C-19 -methyl group, reduction of the C-3 ketone to a hydroxyl group, and the formation of an aromatic ring A. Estradiol is the major estrogen.